Tumor-associated Macrophages Serve as an Acetate Reservoir to Drive Hepatocellular Carcinoma Metastasis
A collaborative research by Dr. LU Ming’s group from the Shanghai Institute of Nutrition and Health of the Chinese Academy of Sciences, and researchers from Huashan Hospital of Fudan University revealed that, in hepatocellular carcinoma (HCC), cancer cells trigger acetate secretion from tumor-associated macrophages (TAMs) through a metabolic interaction involving lactate, the lipid peroxidation–aldehyde dehydrogenase 2 (ALDH2) pathway and acetate. This work has been published in Nature Metabolism on Oct. 20, 2025.
Metabolic reprogramming fuels cancer progression and metastasis. Acetyl-coenzyme A (acetyl-CoA) is a pivotal metabolic intermediate in catabolism of glucose, lipid and amino acids, biosynthesis of lipids, and the TCA cycle, also functions as a signaling molecule due to its role in lysine acetylation. Increased acetyl-CoA production is characteristic of metastatic cancers. To sustain high acetyl-CoA levels, cancer cells actively uptake acetate for acetyl-CoA biosynthesis. Although blood is the primary nutrient source for cancer cells, acetate levels in blood are significantly lower than in cancer tissues, suggesting the presence of acetate-producing cells within the cancer microenvironment. However, the exact source of acetate in the microenvironment remained unclear.
To identify acetate-producing cells in the HCC microenvironment, by utilizing primary TAMs and TAMs derived from cell lines, researchers revealed that TAMs specifically promote acetate accumulation in HCC cells. In an orthotopic HCC mouse model, depletion of TAMs markedly decreased the intracellular acetate levels in HCC cells, highlighting TAMs as a crucial source of acetate in the cancer microenvironment.
Mechanistic investigations employing metabolomics and isotope tracing demonstrated that TAMs generate and secrete acetate through the lipid peroxidation-ALDH2 pathway, and HCC cells subsequently pick up acetate to synthesize acetyl-CoA, which in turn promotes histone H3 acetylation and epithelial-mesenchymal transition, thereby enhancing metastasis.
In vitro studies revealed that inhibiting ALDH2 or lipid peroxidation in TAMs effectively abrogates acetate-induced migration of HCC cells. In an orthotopic HCC mouse model, genetic ablation of Aldh2 in TAMs significantly reduced acetate levels in HCC cells and diminished HCC lung metastasis.
Further investigation demonstrated that lactate secreted by HCC cells acts as an upstream signal to activate the lipid peroxidation-ALDH2 pathway in TAMs by increasing ROS levels.
This study reports a novel metabolic interaction between HCC cells and TAMs,offering new mechanistic insights into how the local metabolic microenvironment influences phenotypic plasticity of cancer cells. It also identifies the lipid peroxidation pathway as a key source of acetate in TAMs, which provide important basis for identifying the lipid peroxidation-ALDH2 pathway in TAMs as target for anti-HCC metastasis.
This work was supported by grants from National Key R&D Program of China and National Natural Science Foundation of China.
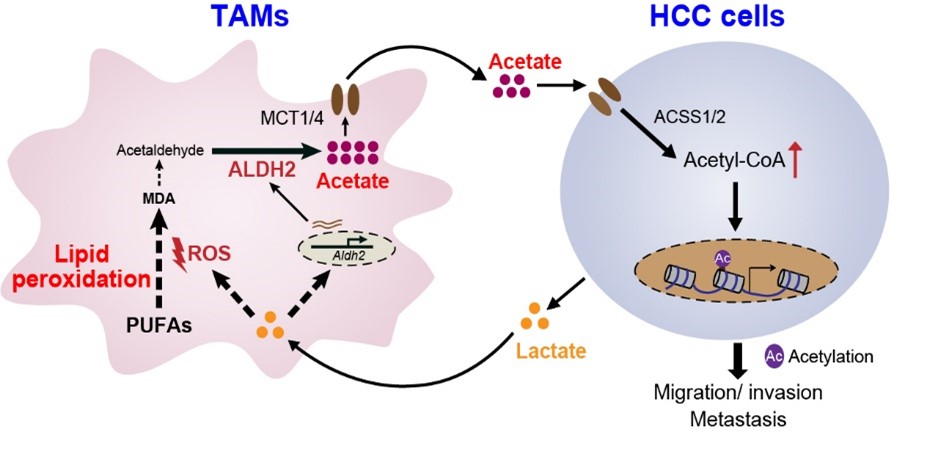
A schematic diagram depicts themetabolic interaction between HCC cells and TAMs.
(Image by Dr. LU Ming’s group)
Paper link: https://www.nature.com/articles/s42255-025-01393-9
Scientific Contact:
Prof. LU Ming
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: mlu@sinh.ac.cn
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn