Researchers Reveal New Mechanism by Which Neutrophil Senescence Influences Sexual Dimorphism in Tumors
Research teams led by Prof. XIAO Yichuan from the Shanghai Institute of Nutrition and Health (SINH), Chinese Academy of Sciences (CAS) and collaborators uncovered a novel mechanism explaining sexual dimorphism in bladder cancer through gut microbiome-modulated neutrophil aging. This research, entitled "Microbiota-shaped neutrophil senescence regulates sexual dimorphism in bladder cancer" was published in Nature Immunology on April 11, 2025.
Clinical statistics indicate that almost all non-reproductive system tumors exhibit sexual dimorphism, primarily manifested as significantly higher tumor incidence and mortality rates in males than in females. Although sex hormones (such as androgens), sex chromosomes, and related genes are considered potential causes, the immunological mechanisms underlying tumor sexual dimorphism remain unclear. The infiltration of various types of immune cells in the tumor microenvironment (TME) is crucial for tumor immune regulation and tumor development. Among these, infiltrating neutrophils play a dual role in tumor immune regulation, exhibiting both pro-tumor and anti-tumor effects. However, whether their functional heterogeneity contributes to tumor sexual dimorphism remains unclear.
Through comprehensive single-cell RNA sequencing analysis of tumor-infiltrating immune cells in male and female mice, the researchers found that neutrophil populations displayed the most pronounced sexual dimorphism among all immune cell types examined. Specifically, they identified a unique senescent-like neutrophil subset characterized by elevated expression of Retnlg and Lcn2 markers, which they designated as Retnlg+Lcn2+ senescence-like neutrophils (RLSNs). These RLSNs exhibited significantly enhanced immunosuppressive activity compared to their non-senescent counterparts and were strikingly enriched in male tumor microenvironments. This male-biased accumulation of RLSNs correlated with substantially diminished anti-tumor immune responses in male subjects. The researchers employed a sophisticated genetic approach using the Retnlg-DTR system to selectively eliminate RLSNs, which resulted in complete abolition of the observed gender disparity in tumor growth rates. This critical experiment provided definitive evidence that neutrophil senescence plays a causal role in mediating tumor sexual dimorphism.
Building on previous research suggesting that neutrophil senescence is regulated by the gut microbiota, the team further discovered that the abundance of Alistipes shahii (A. shahii) was significantly higher in female mice, and the level of its associated metabolite, lurasidone, was also elevated in females. Lurasidone directly targets LCN2, a marker protein of RLSNs. Since LCN2 is an iron sequestrator, this interaction releases intracellular Fe²⁺ and promotes ferroptosis in RLSNs, ultimately clearing RLSNs enriched in female tumors. In contrast, males lack sufficient A. shahii and lurasidone, leading to suppressed ferroptosis in RLSNs and their accumulation in the male tumor microenvironment, thereby inhibiting anti-tumor immunity. Additionally, the accumulated RLSNs in males secrete large amounts of LCN2 protein, which clears iron-sensitive A. shahii, further suppressing its abundance in males.
In summary, this study uncovered a new mechanism involving the microbiota-lurasidone-LCN2 circuit, which contributes to the sexual disparity in bladder cancer, offering a molecular basis for sex-specific therapies. Importantly, the FDA approved antipsychotic drug lurasidone may be repurposed to enhance antitumor immunity either as monotherapy or in combination with existing immunotherapies, presenting a potential new treatment option for male bladder cancer patients.
This research was supported by the grants from CAS Project for Young Scientist in Basic Research, the National Natural Science Foundation of China, the National Key R&D Program of China, Yishan Research Project of Jiangsu Cancer Hospital, etc.
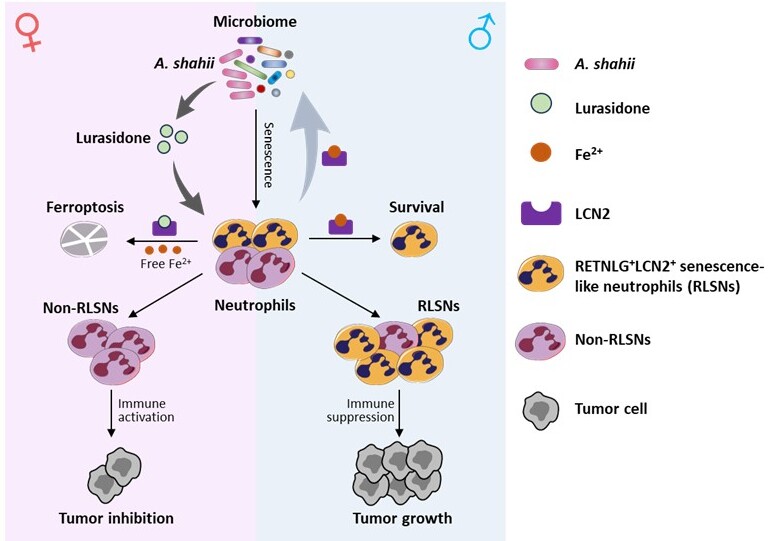
Working model of neutrophil senescence-mediated sexual Dimorphism in tumors.
(Image provided by Prof. XIAO Yichuan's group)
Scientific Contact:
Prof. XIAO Yichuan
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: ycxiao@sinh.ac.cn
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/