Researchers Reveal Sex-Dimorphic Functions of Intestinal MCT1 in Regulating Metabolic Homeostasis
Recently, a team of researchers led by Prof. CHEN Yan from Shanghai Institute of Nutrition and Health (SINH) of Chinese Academy of Sciences revealed for the first time that Intestinal monocarboxylate transporter 1 (MCT1) can regulate glucose homeostasis and energy metabolism in a sex-dimorphic pattern.
As the concept of lactate shuttling between producers and consumers being gradually recognized, lactate has recently been regarded as an important signaling molecule and driver of many biochemical and physiological processes. MCT1, encoded by Slc16a1, is a member of solute carrier family 16 and plays a crucial role in the transportation of lactate, pyruvate, ketone bodies, and short-chain fatty acids. MCT1 is widely distributed in almost all human tissues. In recent years, the functions of MCT1 in different tissues are beginning to be recognized, highlighting MCT1 as an important player in numerous physiological processes and diseases.
Intestine is the most important organ for nutrient digestion and absorption, as well as being considered to be the largest immune organ. However, the role of intestinal MCT1 in regulating lactate transport and modulating metabolism of the body is unclear.
In this study, the researchers generated a mouse model with specific deletion of Slc16a1 in intestinal epithelium and investigated the functions of MCT1 in the gut. When fed with a high-fat diet, Slc16a1-deleted male mice had improvement in glucose tolerance and insulin sensitivity, while Slc16a1-deleted female mice only had increased adiposity. Deficiency of intestinal MCT1 in male mice was associated with downregulation of pro-inflammatory pathways, together with decreased circulating levels of inflammatory cytokines including TNF-α and CCL-2. Intestinal deletion of Slc16a1 in male mice reduced interstitial lactate level in intestine. Lactate had a stimulatory effect on pro-inflammatory macrophages in vitro. The number of intestinal macrophages was reduced in Slc16a1-deleted male mice in vivo.
In addition, treatment with sex hormone estrogen could abolish the difference of glucose homeostasis between Slc16a1-deleted and wild type male mice and produce an impact on lactate efflux in the intestines. Deficiency of intestinal MCT1 also blocked transport of lactate and short chain fatty acids from the intestine to the portal vein. The effect of Slc16a1 deletion on glucose homeostasis in male mice was partially mediated by alterations in gut microbiota.
In summary, this work revealed that intestinal MCT1 regulates metabolism of the body in a sex-dependent manner. These findings further corroborated the concept that metabolic homeostasis is differently regulated in two sexes. This work also strengthened the demand for developing sex-specific medicines for metabolic disorders and highlighted the importance of stratifying patients based on sexes in the management and treatment of metabolic diseases.
The research article entitled “Intestinal monocarboxylate transporter 1 mediates lactate transport in the gut and regulates metabolic homeostasis of mouse in a sex-dimorphic pattern” was published online in Life Metabolism on Nov. 6, 2023. The assistant research fellow Dr. WANG Shuo at SINH is the first author of this article.
This work was supported by National Natural Science Foundation of China and Shanghai Municipal Science and Technology Major Project, with acknowledgement of the assistance from the public testing technical platform and institutional animal facility of SINH.
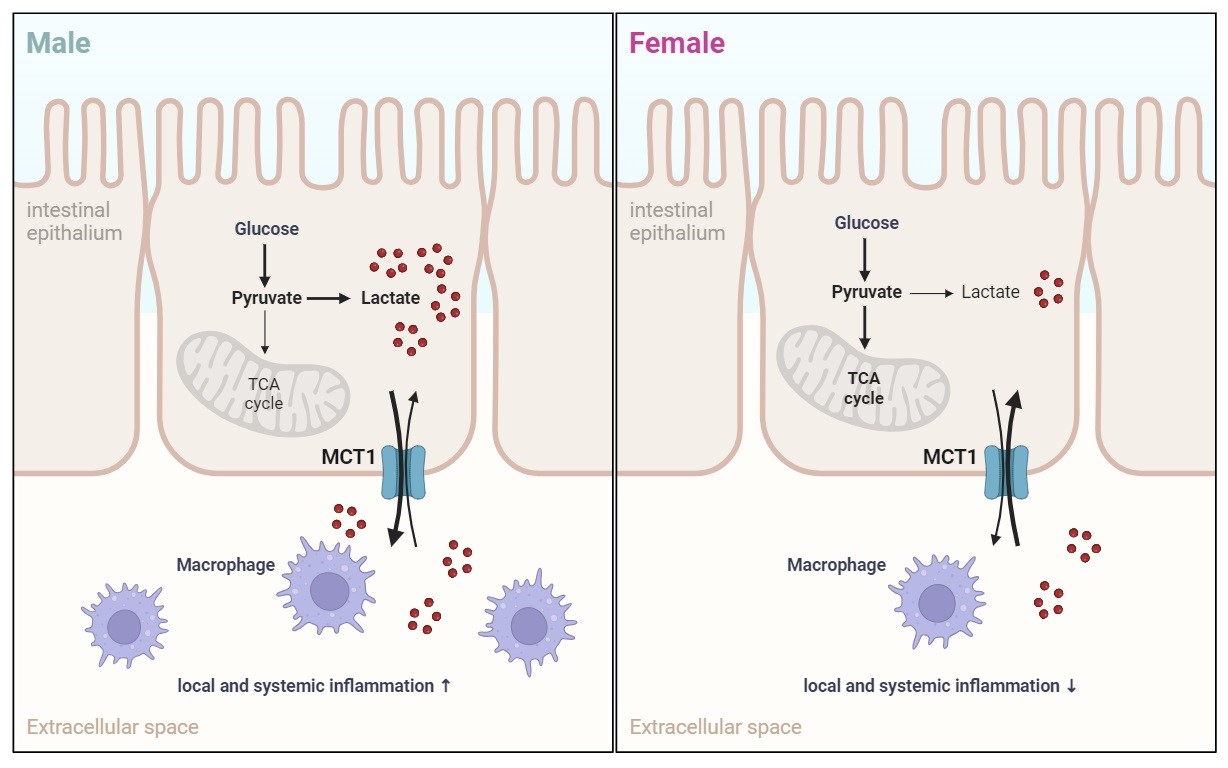
MCT1 regulates metabolic homeostasis of mouse in a sex-dimorphic pattern. (Image provided by Prof. CHEN Yan’s group)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/
