Chinese Researchers Reveal New Mechanism in Regulation of Breast Cancer Lung Metastatic Dormancy
A research group led by Dr. HU Guohong from the Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences revealed that NR2F1-AS1, a long non-coding RNA, facilitates breast cancer lung metastatic dormancy by regulating NR2F1 andΔNp63.
Metastasis of breast cancer which accounts for most patients’ death demonstrates a serious threat to women’s health. In the process of metastasis, disseminated tumor cells in the distant organs usually fall into a slow proliferation, sustained survival, and therapy-resistant state which is termed metastatic dormancy. Dormant tumor cells could exist even decades with being undetectable and the capacity of reactivation to form tumor recurrence. Previous studies have noticed that dormant tumor cells retain the new tumor-initiating capacity after a long period of quiescence, which is a decisive feature of Cancer Stem-like Cells (CSCs). However, the current understanding of the relationship between metastatic dormancy and cancer stemness is not consistent. There are two independent CSC populations in breast cancer: mesenchymal-like CSC (M-BCSC) and epithelial-like CSC (E-BCSC). Interestingly, E-BCSC is more proliferative and tumorigenic than M-BCSC, and M-BCSC tends to be quiescent. These studies indicated that the inconsistency in the role of CSCs in metastatic dormancy may be explained by the heterogeneity of CSCs.
This study led by Dr. HU Guohong showed that M-BCSC is more easily disseminated to distant organs from primary tumor than E-BCSC, but disseminated E-BCSC in the lung is more likely to form macro-metastases, while M-BCSC tends to be quiescent in the lung. Transcriptomic analysis revealed that long non-coding RNA NR2F1-AS1 was highly expressed in dormant M-BCSC. Functional studies have shown that NR2F1-AS1 enhanced M-BCSC properties by promoting EMT of tumor cells facilitating the dissemination of tumor cells in primary tumor and inhibited E-BCSC properties impairing the reactivation capacity of tumor cells in the lung. And NR2F1-AS1 showed a metastatic dormancy-promoting function, ultimately. Mechanically, NR2F1-AS1 facilitates the contact between PTBP1 and the 5’ UTR of NR2F1, and PTBP1 promotes NR2F1 translation by recruiting ribosomes to the Internal Ribosome Entry Site (IRES) in 5’ UTR of NR2F1. Furthermore, NR2F1 impairs the properties of E-BCSC and enhances M-BCSC properties by inhibiting the expression of ΔNP63. Analyses of patient-derived tumor samples also confirmed that NR2F1-AS1 is significantly correlated with metastatic dormancy and BCSC properties.
In summary, this study elucidated the functional role and mechanisms of NR2F1-AS1 in the regulation of breast cancer lung metastatic dormancy and explained the role of CSCs in metastatic dormancy from the perspective of BCSCs heterogeneity. The theory demonstrated by this study deepens the understanding of metastatic dormancy. Entitled “Long non-coding RNA NR2F1-AS1 induces breast cancer lung metastatic dormancy by regulating NR2F1 and ΔNp63”, this research was published online in Nature Communications on Sep 2nd, 2021.
Dr. HU Guohong is the corresponding author of the article. Dr. LIU Yingjie and Dr. ZHANG Peiyuan from SINH are the co-first authors of the work. The work was also supported by Dr. LIAO Lujian at East China Normal University, Dr. YANG Qifeng at Qilu Hospital of Shandong University, Dr. YANG Qingcheng at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, and Dr. QIN Lunxiu at Huashan Hospital of Fudan University. This project was sponsored by the Chinese Academy of Sciences, the Ministry of Science and Technology of China, the National Natural Science Foundation of China, and the Postdoctoral Science Foundation of China.
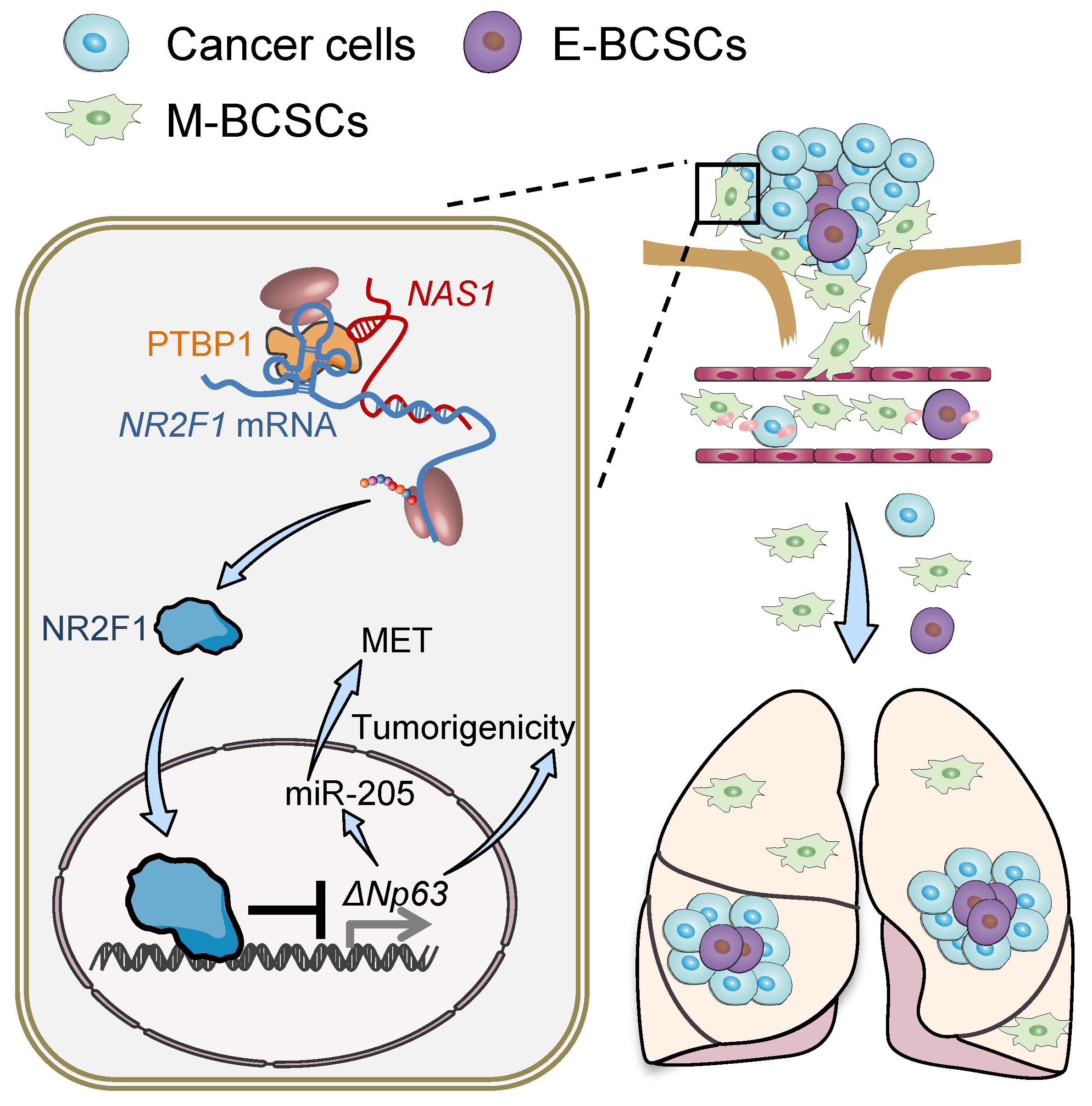
Schematic model of the role of NR2F1-AS1 in breast cancer lung metastatic dormancy regulation.
(Image by Dr. HU Guohong's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
Web: http://english.sinh.cas.cn/
