ALDOB Depletion Promotes Hepatocellular Carcinogenesis Through Activating Insulin Receptor Signaling and Lipogenesis
A recent study led by Prof. YIN Huiyong from the Shanghai Institute of Nutrition and Health (SINH), of the Chinese Academy of Sciences (CAS), revealed that Fructose-1,6-bisphosphate aldolase B (ALDOB) directly binds with insulin receptor (IR) to attenuates IR nuclear translocation, p-AKT and de novo fatty acid synthesis in hepatocellular carcinogenesis (HCC). This work was published online in Hepatology on July 22, 2021, entitled “ALDOB Depletion Promotes Hepatocellular Carcinogenesis through Activating Insulin Receptor Signaling and Lipogenesis”.
Insulin receptor signaling is a central pathway in glucose and lipid metabolism: upon insulin binding, IR transduces cell surface signal via canonical phosphoinositide-3-kinase (PI3K)-AKT pathway or translocate to the nucleus and binds to the promoters to regulate gene expressions associated with insulin action. Dysregulated IR signaling may cause glucose and lipid metabolism disorders, such as diabetes mellitus, fatty liver, cardiovascular diseases, and various cancers, including HCC. As a primary liver malignancy, the global incidence and mortality rate of HCC rank as the 6th and 4th respectively. However, it remains poorly defined how IR signaling pathway and its related metabolic reprogramming are involved in HCC.
ALDOB is a member of fructose-1,6-bisphosphate aldolases that catalyzes the conversion of FBP to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate in glycolysis and the reversible reaction in gluconeogenesis.
In this study, researchers found that ALDOB physically interacts with IR and attenuates IR signaling through downregulating PI3K-AKT pathways and suppressing IR nuclear translocation. ALDOB depletion or disruption of IR/ALDOB interaction in ALDOB mutants promotes DNL and tumorigenesis, which is significantly reversed with ALDOB restoration in L-ALDOB-/- mice.
Notably, attenuated IR/ALDOB interaction in ALDOB-R46A mutant exhibits more significant tumorigenesis than releasing ALDOB/AKT interaction in ALDOB-R43A, while knockdown IR sufficiently diminishes tumor-promoting effects in both mutants. Furthermore, inhibiting p-AKT or fatty acid synthase significantly attenuates HCC in L-ALDOB-/- mice. Consistently, ALDOB down-regulation is correlated with upregulation of IR signaling and DNL in human HCC tumor tissues.
In conclusion, the study revealed a mechanism by which loss of ALDOB activates IR signaling primarily through releasing IR/ALDOB interaction to promote DNL and HCC, and highlighted a potential therapeutic strategy in HCC.
Prof. YIN Huiyong and Dr. TAO Yongzhen from SINH are co-corresponding authors for this work. Ph.D. graduate student Ms. LIU Guijun from Prof. Yin’s laboratory at SINH is the lead author for this work. Clinical data in this work was supported by Drs. LI Nan and ZHANG Cunzhen of the Shanghai Eastern Hepatobiliary Surgery Hospital. This work was financially supported by the National Natural Science Foundation of China and the Ministry of Science and Technology of PRC.
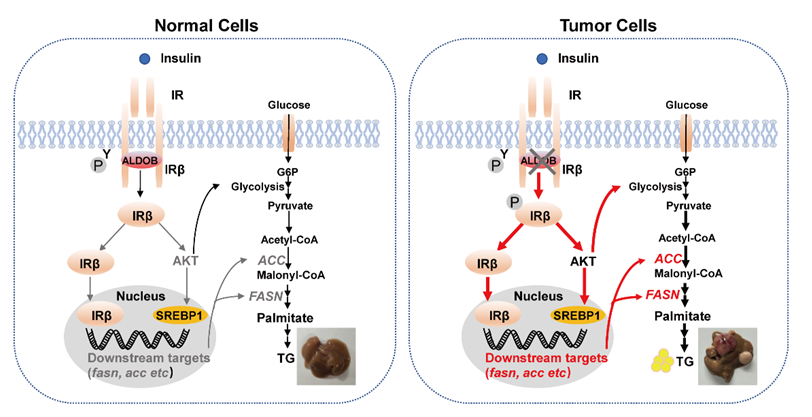
ALDOB/IR interaction regulates IR signaling and de novo fatty acid synthesis in HCC.
(Image provided by Prof. YIN Huiyong's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
Web: http://english.sinh.cas.cn/
