Scientists Reveal Novel Mechanism Controlling TH9-mediated Cancer Immunotherapy
On April 29th, 2021, the research article “BFAR coordinates TGFb signaling to modulate Th9-mediated cancer immunotherapy” was published online in Journal of Experimental Medicine. This work discovered that high concentration of TGFβ inhibits the expression of BFAR in CD4+ T cells, so as to impair TH9 differentiation as well as Th9-mediated tumor immunotherapy and elucidates the underlying molecular mechanism. The research was accomplished by Dr. XIAO Yichuan’s group from Shanghai Institute of Nutrition and Health (SINH), of the Chinese Academy of Sciences (CAS) , and their collaborators.
As an emerging and effective clinical treatment for tumor, immunotherapy has become one of most promising directions in the global clinical treatment, especially in patients with tumor recurrence, chemotherapy tolerance, unresectable tumor and other clinical problems. In recent years, PD-1/PD-L1-blocking therapy has been used to cure several kinds of tumors through promoting anti-tumor immune response. However, this treatment is effective in only a small subset of people. Therefore, the study of the mechanism of insensitivity of this therapy is the key to improve the effect of immune checkpoint therapy.
Worked as an immune suppressor, TGFβ has been reported to be highly-expressed in multiple kinds of tumor tissues and is able to regulate tumor immune escape. As a result, the blockage of TGFβ signaling pathway would enhance the effect of tumor immunotherapy, but the underlying mechanism still needs to be elucidated.
Th9 cells are a subset of CD4+ T cells with obvious anti-tumor effect, which can not only effectively kill and clear solid tumors, but also inhibit tumor metastasis and recurrence. TGFβ is known to induce Th9 differentiation, and whether TGFβ1 could promote TH9 cells differentiation and exhibit anti-tumor effect is not clear.
Unexpectedly, the researchers found that high concentration of TGFβ1 in plasma from tumor patients was inversely correlated with IL-9 levels. High level TGFβ1 can negatively regulate the activation of TGFβ signaling pathway and TH9 cell inducibility by inhibiting the expression of BFAR, and thus impairing anti-tumor immune response. Mechanistically, BFAR mediates K63-linked ubiquitination of TGFβR1 at K268, which is critical to activate TGFβ signaling. Thus, BFAR deficiency or K268R knock-in mutation suppresses TGFβR1 ubiquitination and TH9 differentiation, thereby inhibiting TH9-meidated cancer immunotherapy.
More importantly, BFAR-overexpressed TH9 cells exhibited promising therapeutic efficacy to curtail tumor growth and metastasis in mouse cutaneous melanoma model and the lung metastasis model, and increased the sensitivity of the PD-1 antibody mediated immunotherapy. Moreover, the researchers generated a patient-derived xenograft (PDX) colorectal tumor model in immunodeficient NCG mice and found BFAR-OE TH9 cells showed ideal effect in eliminating tumor growth.
Overall, this finding discovered BFAR as a key TGFβ1-regulated gene to fine-tune TGFβ signaling activity and TH9 induction, which provides a new strategy for enhancing the therapeutic efficacy of TH9 mediated tumor immunotherapy.
This research was funded by the grants from the National Key R&D Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of CAS, the Key Research Program of CAS, Zhejiang Provincial Medical and Health Science Foundation, and CAS Key Laboratory of Tissue Microenvironment and Tumor.
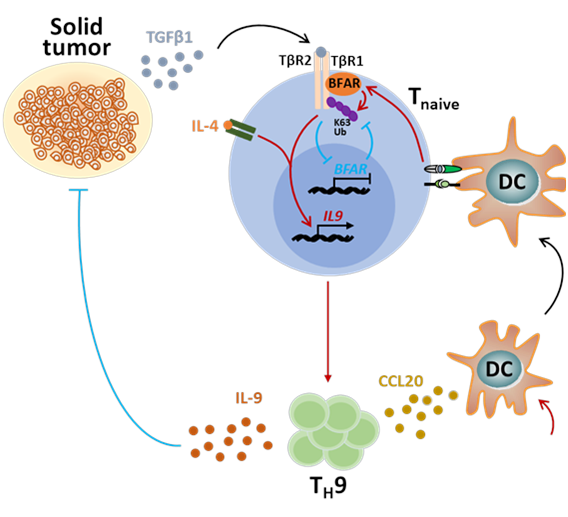
Working model of BFAR modulating TH9-meidated cancer immunotherapy.
(Image provided by Dr. XIAO Yichuan's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
Web: http://english.sinh.cas.cn/
