The Glycolytic Enzyme Aldolase B Acts as a Metabolic Switch for Metabolic Reprogramming in Hepatocellular Carcinogenesis
Metabolic reprogramming is one of the most important hallmarks of cancer. Tumor cells undergo a series of metabolic reprogramming to meet the demands for rapid proliferation and growth. The Warburg Effect proposed by Otto Warburg in the 1920s points out that even under aerobic conditions, tumor cells prefer anaerobic glycolysis to produce energy and biosynthetic building blocks for cell proliferation. Since then, tremendous research efforts have been made to understand the underlying molecular mechanisms of metabolic reprogramming and its clinical significance.
Recent studies have unveiled a diverse mechanisms for cancer cells to achieve metabolic reprogramming, such as gene mutations, epigenetic alterations, protein-protein interactions, and the interaction between metabolic enzymes and metabolites. Aldolase catalyzes the cleavage of fructose-1,6-bis-phosphate (FBP) to generate dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) in glycolysis, as well as the reverse reaction of DHAP and GAP to FBP in gluconeogenesis. Aldolase B (Aldob) is abundantly expressed in the liver, small intestine, and kidney. Previous studies have found that Aldob is down-regulated in liver cancer tissues and its expression is reversely correlated to clinical prognosis for liver cancer patients. However, the molecular mechanisms and clinical significance of Aldob downregulation in hepatocellular carcinoma (HCC) remain to be defined.
On July 6, 2020, Nature Cancer online published a research paper entitled "Aldolase B Suppresses Hepatocellular Carcinogenesis by Inhibiting G6PD and Pentose Phosphate Pathways" from Dr. YIN Huiyong's group at the Shanghai Institute of Nutrition and Health (SINH), of the Chinese Academy of Sciences(CAS). This study discovers a novel mechanism by which Aldob regulates liver cancer metabolic reprogramming.
In this study, the researchers started out from gene expression microarray analysis of paired human HCC tissues and found that the expression of Aldob was significantly downregulated, whereas glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the pentose phosphate pathway (PPP), was significantly upregulated in tumor tissues compared to peripheral normal tissue. Furthermore, a low expression of Aldob and high G6PD expression was positively associated with short overall survival and poor prognosis for HCC patients.
A multivariate analysis demonstrated that Aldob is an independent risk factor for HCC. Using N-Nitrosodiethylamine (DEN)-induced HCC mouse model, they further found that global or liver specific knockout (KO) of Aldob promotes hepatocellular carcinogenesis through enhancing G6PD activity to increase the metabolic flux to PPP, while re-expression of Aldob in liver-specific Aldob KO mice suppresses HCC. Consistently, pharmacological inhibition or genetic knocking down G6PD attenuates the tumor formation. All these in vivo data suggest that Aldob opposes HCC through regulation of G6PD and PPP.
They further demonstrated that in normal liver cells, Aldob interacts with G6PD to inhibit its activity, and the presence of Aldob enhances the inhibitory effect of tumor suppressor p53 on G6PD through a protein complex containing Aldob, G6PD and p53 to regulate cell metabolism. In cancer cells, however, Aldob downregulation releases G6PD activity and attenuates the inhibitory effect of p53 on G6PD, leading to the increased metabolism of PPP to meet the need for rapid growth of cancer cells.
In summary, this study has defined a new molecular mechanism for cancer cell metabolic reprogramming: the interaction of metabolic enzymes Aldob and G6PD regulates cell metabolism in glycolysis and pentose phosphate pathways, whereas the loss of Aldob in tumor cells leads to rewire metabolic pathways to favor tumor growth. The study found a new non-enzymatic function of Aldob, highlighting a clinical significance in diagnosis and treatment of liver cancer.
The graduate students Drs. LI Min and HE Xuxiao from SINH, and Dr. GUO Weixin from The Eastern Hepatobiliary Surgery Hospital, Shanghai, are the co-first authors for this work. Prof. YIN Huiyong is the lead contact for this article. Dr. TAO Yongzhen, a senior research assistant from SINH, and Dr. CHENG Shuqun, a chief physician from The Eastern Hepatobiliary Surgery Hospital are designated as co-corresponding authors. The authors acknowledged the help from Dr. LI Dangsheng and Prof. DING Jianping from the Center for Excellence in Molecular Cell Science of CAS, Prof. LI Yu from SINH, and Prof. LIN Shengcai and LIN Shuhai from Xiamen University. This work was funded by the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, and CAS.
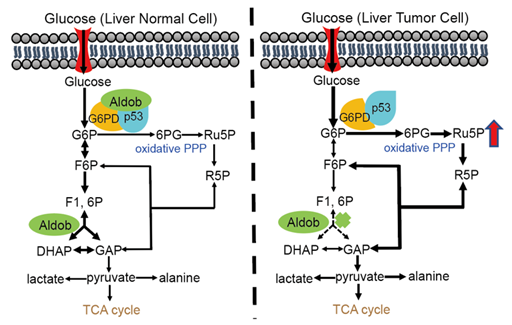
Aldob inhibits liver cancer by directly interacting with G6PD and enhancing p53-mediated inhibition of G6PD (Image provided by Prof. YIN Huiyong's Group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
