Researchers Reveal Epigenetic Switch in Regulating Rhythmic Chromatin Remodeling and Lipid Synthesis in Liver
A recent study, published online in Nature Metabolism, revealed an evolutionally highly conserved chromatin remodeler MRG15 as a global epigenetic switch that orchestrates chromatin modification, RNA polymerase II (Pol II) recruitment and gene expression. It enables rhythmic global activation of lipid synthetic genes in the liver, which could be a novel target for liver lipid disorders. This research was performed by Dr. DING Qiurong's research group from Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences.
It is appreciated that the oscillation of circadian rhythms is orchestrated by global circadian regulation of processes such as Pol II recruitment and chromatin remodeling, and is intimately linked to lipid homeostasis. Several publications in the past have well illustrated the major role of Rev-erb/HDAC3/NCoR complex in rhythmic chromatin modifications that result in global transcriptional repressing of lipid synthesis genes, especially when animals are inactive and in starvation
It is reasonable to envisage that, besides inhibition of lipogenesis in starvation to assure enough energy in keeping basal body activity, evolutionally there should be a counterpart complex that works in contrast to the Rev-erb/HDAC3/NCoR complex, which can actively turn on the genome-wide epigenomic switch of lipid synthetic genes, to start lipogenesis at feeding for efficient energy storage. And the researchers discovered in the current study that MRG15/LRH-1 represents such a complex.
The researchers started with comprehensive analysis of the genomic targets of MRG15 in mouse liver. Remarkably, they found that MRG15 displays a significant rhythmic genomic recruitment in mouse liver (10250 peaks at ZT22 vs. 57 peaks at ZT10). The majority of MRG15 binding sites at ZT22 overlap nicely with where Pol II is recruited; and importantly, the rhythmic genome-wide Pol II recruitment is entirely lost upon MRG15 depletion, so do histone acetylation signals near lipid synthetic genes, establishing MRG15 as a key modulator in global rhythmic transcriptional regulation.
They further identified nuclear receptor LRH-1, rather than any known core clock proteins, that mediates the rhythmic MRG15 recruitment to lipid synthesis genes. Moreover, blocking liver MRG15 by CRISPR targeting or treatment with FDA-approved drug argatroban, which is clinically applied as a thrombin inhibitor, but also acts as an antagonist to MRG15, attenuates liver steatosis and improves metabolism when animals are under a high fat diet.
Altogether, this study uncovers a key mechanism, by which animals actively coordinates energy intake with energy storage, at the epigenomic level, to well anticipate energy fluctuations, which is essentially very important in food-scarce seasons to improve survival, however, causes metabolic diseases in overnutrition conditions. Furthermore, the existence of a FDA-approved drug that can target MRG15 and alleviate liver steatosis, also demonstrates that MRG15 can be a potential pharmacological target to treat hepatic lipid disorders.
This study entitled "MRG15 orchestrates rhythmic epigenomic remodeling and controls hepatic lipid metabolism" was published online in Nature Metabolism on May 4, 2020, and was supported by grants from the National Key R&D Program of China, the Strategic Priority Research Program and the Key Research Program of the Chinese Academy of Sciences, the National Natural Science Foundation of China, and CAS Key Laboratory of Nutrition, Metabolism and Food Safety.
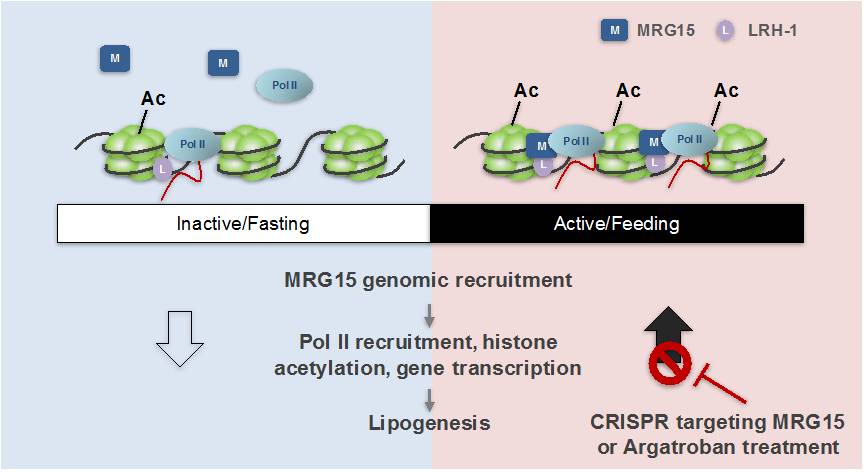
MRG15 acts as an epigenetic switch in regulating rhythmic chromatin remodeling and lipid synthesis in liver. (Image by Dr. DING Qiurong's Group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
