Scientists Reveal the Double Edge of VEGFA Signaling in Vascular Homeostasis
Homeostasis and remodeling of blood vessels interweaved within tissues and organs are tightly controlled by cell metabolism and intracellular signaling network, crosstalk among which determines the specificity and flexibility of final outputs, including vasculogenesis, angiogenesis, pruning and regression. Vascular endothelial growth factor A (VEGFA) has been well recognized by its pro-angiogenic effect for decades. However, how VEGFA signaling synchronizes with different metabolic states in the endothelium in vivo and the implicated molecular mechanism remain poorly understood.
Previously study demonstrated that zebrafish cds2 mutants (an enzyme controls phosphoinositide metabolism) displayed endothelium-specific morphogenic defects with impaired angiogenesis and reduced VEGFA signal activity, which were mainly caused by the insufficiency of phosphoinositide (PIP2) recycling (Pan et al. Blood, 2012).
Recently, the group led by Prof. PAN Weijun from Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences (CAS) found that genetic deletion of CDS2 (CDP-diacylglycerol synthetase-2) converted the outcome of VEGFA signaling from angiogenesis to vascular regression.
Live imaging analysis revealed the presence of reverse migration and cell apoptosis in cds2 mutant zebrafish with VEGFA stimulation. Such vascular regression was further validated in the postnatal retina and implanted tumor models using a Cds2 EC (endothelial cell)-inducible knockout mice. In tumors, CDS2 deficiency blocked tumor angiogenesis, triggered hypoxia and enhanced the level of tumor-secreted VEGFA, which in-turn caused the greater vessel regression, eventually leading to limited tumor growth or regression of tumors. Mechanistically, VEGFA stimulation reduced phosphatidylinositol (4,5)-bisphosphate (PIP2) availability in the absence of CDS2-controlled-phosphoinositide metabolism, subsequently causing phosphatidylinositol (3,4,5)-triphosphate (PIP3) deficiency and finally resulting in FOXO1 (Forkhead box protein O1) activation-mediated vessel regression.
This study thus reveals the double edge of VEGFA signaling in vascular homeostasis and suggests that targeting endothelial CDS2 is a novel anti-tumor vessel strategy, showing great potential on tumor therapy.
A research article describing this work, entitled “Endothelial CDS2 Deficiency Causes VEGFA-mediated Vascular Regression and Tumor Inhibition” was published online in Cell Research on Sep 9, 2019 (https://doi.org/10.1038/s41422-019-0229-5).
Ph.D. student ZHAO Wencao, CAO Le and YING hanru are the co-first authors of this publication. The study was supported by grants from CAS Strategic Priority Research Program, National Natural Science Foundation of China, Ministry of Science and Technology of China, and CAS Scientific Research Equipment Development Project.
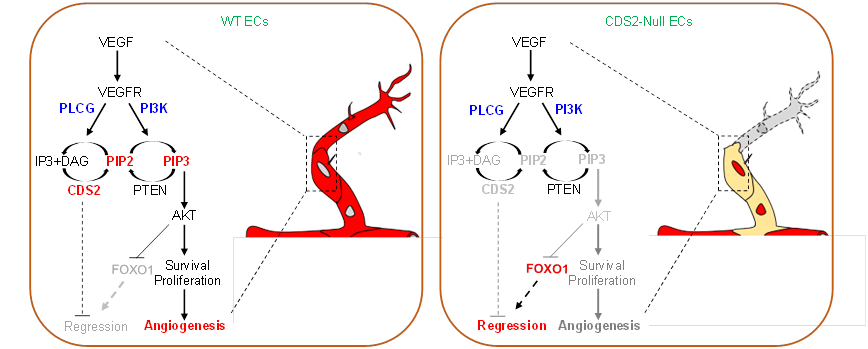
Working model of VEGFA-triggered vessel regression on CDS2 deficient endothelium. The outcome of VEGFA signaling can be reversed from angiogenesis to vessel regression, which is dependent on CDS2-controlled PIP2 and PIP3 availability and FOXO1 signaling activation. (Image by Prof. Pan's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
