Scientists Identify New Molecular Mechanism for Type 2 Diabetes Susceptibility at the SLC16A11 Locus
Human genetic studies, e.g. genome wide association studies (GWAS), have generated a wealth of data in just the last few years. A large number of genetic loci associated with different human diseases have been identified across the genome. However, challenges remain in identifying the biological mechanisms at work at each of these loci. A recent study led by Dr. DING Qiurong from Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences identified that genetic variants associated with type 2 diabetes (T2D) in the SLC16A11 coding region produced a gain-of-function mutant protein that affects lipid metabolism in liver and leads to the progression of type 2 diabetes.
The genetic variants at the SLC16A11 locus were previously identified in GWAS in the Mexican population to be significantly associated with T2D (Williams, A. L., et al. Nature, 2014). The risk haplotype carries four missense SNPs that are found in the SLC16A11 gene, which encodes a poorly characterized member of the monocarboxylic acid transporter family of solute carriers. How these variants directly contribute to metabolic disorders remains unclear.
A study published in 2017 identified that SLC16A11 is an H+-coupled monocarboxylate transporter localized in cell surface (Rusu, V., et al. Cell. 2017). The study pointed out that the genetic variants identified in the SLC16A11 coding region caused disrupted SLC16A11 surface localization, which lead to reduced function of SLC16A11 and consequently metabolic changes associated with increased T2D risk. Therapeutics that enhance SLC16A11 levels or activity were therefore proposed to treat T2D. However, these studies mainly focus on analysis of human genetic association studies with validation performed with in vitro cultured cells, lacking experimental evidences that address the direct causality in a more physiologically relevant setting.
In the newly published study, with the aim to directly examine the contribution of SLC16A11 in metabolic regulation, researchers generated a Slc16a11 knockout mice; on the basis of the knockout mouse model, they further studied the potential influence of the coding variants identified in human genetics by reconstitution of the mutant SLC16A11 protein expression carrying these variants in knockout mouse livers. In contradictory to the previous study (Rusu,V., et al. Cell. 2017), they found no metabolic defects in the Slc16a11 knockout mice; instead, reconstitution of the mutant SLC16A11 protein, but not the wild-type protein, caused significant lipid accumulation in mouse liver and in blood, and induction of insulin resistance in these animals. Further studies pointed out that the mutant SLC16A11 protein can cause significant upregulation of Lipin1 expression in liver, which is actively involved in lipid synthesis. Increased Lipin1 expression consequently lead to more lipid accumulation in liver and development of insulin resistance.
Altogether, this study showed that the genetic variants in the SLC16A11 locus produced a mutant protein that obtains aberrant functions, and provided a different explanation to the function of these diabetic variants, challenging the concept of enhancing SLC16A11 function to treat T2DM. The contradictory results by this and the previous studies suggest that how the SLC16A11 locus contributes to human metabolism warrant further investigation.
This work has been published online in Cell Reports on Jan 22th, 2019 entitled “Gain-of-function mutations of SLC16A11 contribute to the pathogenesis of type 2 diabetes”. This study was funded by the grants from National Key Research and Development Program and the Strategic Priority Research Program of the Chinese Academy of Sciences, etc.
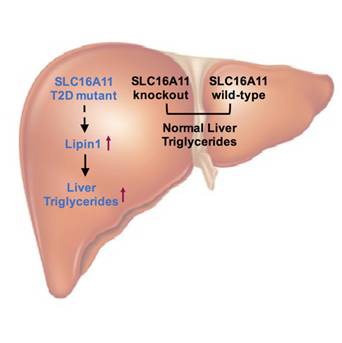
Scientists show that genetic variants associated with type 2 diabetes (T2D) in the SLC16A11 coding region produce a gain-of-function mutant protein that affects lipid metabolism in liver and leads to the progression of type 2 diabetes. (Image by Dr. Ding Qiurong's Group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
