- Home >> ALL News >> Highlights
Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity
A team of Chinese scientists have shown the unique structure of circular RNAs (circRNAs) and their binding and regulation of the innate immune double-stranded RNA(dsRNA)-activated protein kinase (PKR). Importantly, misregulation of circRNA on PKR is found in patients with autoimmune disease. This work entitled “Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity” was published in Cell on May 02, 2019 (DOI:
A large number of circRNAs produced from precursor mRNA (pre-mRNA) back-splicing of exon(s) have been identified in eukaryotes with non-linear conformation and lack of polyadenylated (poly(A)) tails (Li et al, Mol Cell 2018). By developing and applying a series of new RNA-seq strategies and specific computational pipelines for circRNA analyses, Dr. YANG Li’s lab has shown that circRNAs are widely expressed in transcriptomes from different species and their biogenesis can be modulated by both intronic complementary sequences (ICSs) and RNA binding proteins (RBPs) (Zhang et al, Cell 2014; Zhang et al, Genome Res 2016; Zhang et al, Cell Rep 2016; Dong et al, RNA Biol 2017; Li et al, Mol Cell 2017; etc). However, due that circRNAs contain sequences almost entirely identical to those of their linear cognate RNAs, challenges exist at multiple levels to understand their regulation and functions (Li et al, Mol Cell 2018). For example, circRNAs are stable due to their circularity and resistance to the cellular linear RNA decay machineries. So far, little is known how circRNAs are degraded in cells. In addition, the circular feature may endow circRNAs with unique structural conformations to distinguish them from linear RNAs. However, the detection of circRNA structures has been hindered by largely overlapping sequences between circRNAs and their linear cognate RNAs. In this scenario, deciphering how circRNAs are degraded and structured will help to understand their underlying functions.
The recent published study shows that endogenous circRNAs tend to form 16–26 bp imperfect RNA duplexes and act as inhibitors of PKR related to innate immunity. Upon poly(I:C) stimulation or viral infection, circRNAs are globally degraded by RNase L, a process required for PKR activation in early cellular innate immune responses. Augmented PKR phosphorylation and circRNA reduction are found in peripheral blood mononuclear cells (PBMCs) derived from patients with autoimmune disease systemic lupus erythematosus (SLE). Importantly, overexpression of the dsRNA-containing circRNA in PBMCs or T cells derived from SLE can alleviate the aberrant PKR activation cascade, thus providing a connection between circRNAs and SLE. Notably, although the causality between circRNA expression, RNase L and PKR activation, and SLE still requires further investigation, these most recent progresses uncover an unexpected pathophysiological connection of circRNA function to autoimmune diseases.
This study was supported by grants from Chinese Academy of Sciences, the National Natural Science Foundation of China, Ministry of Science and Technology, and Howard Hughes Medical Institute International Program.
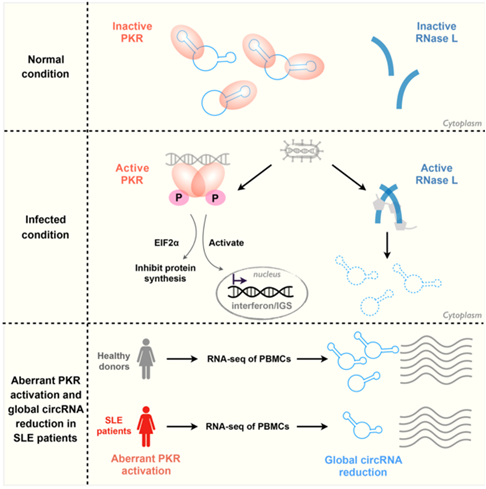
Structure and degradation of circular RNAs regulate PKR activation in innate immunity.
(Image provided by Dr. Yang's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn