Researchers Discover AMBRA1 Regulating T Cell Death at Translational Level
A study led by Prof. YAO Yikun’s group from the Shanghai Institute of Nutrition and Health (SINH), Chinese Academy of Sciences (CAS) identified and characterized the key factor of Activating Molecule in BECN1-regulated Autophagy Protein 1 (AMBRA1) in the FAS-mediated T cell death process. Researchers revealed a new translation dependent mechanism by which AMBRA1 controls TCR signaling, T cell cycle, and T cell death.
After antigen stimulation of the T cell receptor, T cells are rapidly activated and differentiate into effector T cells or memory T cells. Activated T cells quickly initiate glycolysis to generate energy and essential components for T cell proliferation, requiring the rapid synthesis of a large amount of protein. Therefore, in addition to transcriptional regulation, the regulation of translation at a more immediate level may also play a significant role in T cell activation. Following T cell activation, amino acid uptake increases rapidly, but mRNA levels do not show a corresponding increase, suggesting that the main driving factor for the increased protein synthesis in activated T cells is likely translation rather than transcription. Previous studies on T cell activation and activation-induced T cell death have largely focused on transcriptional regulation, with insufficient exploration of translation-level regulation in T cells.
To uncover new regulatory factors mediating T cell death, researchers utilized a whole-genome CRISPR screening and discovered the scaffold protein AMBRA1 plays a crucial role in FAS-mediated T cell death. AMBRA1 is an evolutionarily conserved scaffold protein. Previous studies have shown that AMBRA1 is a key regulatory factor in autophagy, E3 ubiquitin ligase activity, and cyclin proteins. However, the function of AMBRA1 and its translation-related regulatory mechanisms in lymphocytes is unknown.
Researchers performed flow cytometry, Western blotting, and a series of functional rescue experiments to verify that the knockout of AMBRA1 reduces FAS protein expression and inhibits FAS pathway-induced T cell apoptosis. In further mechanistic investigations, the researchers utilized OPP incorporation combined with click chemistry, sucrose gradient centrifugation to isolate ribosomes and their associated mRNA, and other techniques. They found that AMBRA1 promotes the translation of FAS mRNA, a regulatory effect that is crucial for the complete expression of FAS following T cell activation.
Through RNA-seq and mass spectrometry, researchers found TCR signaling significantly stimulates the expression of AMBRA1. Further investigations using TCR downstream pathway inhibitors and an AMBRA1 5' UTR reporter gene revealed the existence of a translation control circuit induced by TCR stimulation. This circuit can enhance the translation of AMBRA1 after T cell activation via the CD28–PI3K–mTORC1–eIF4F axis, thereby regulating the expression of FAS and other immune-related genes.
Subsequently, researchers used a series of protein interaction and translation level detection techniques, such as Proximity-dependent Biotinylation (BioID), The Surface Sensing of Translation assay (SunSET), and Multiplexed Enhanced Protein Dynamics Proteomics (mePROD), which indicated that AMBRA1 interacts with multiple ribosomal proteins and promotes the translation of various ribosome biogenesis-related proteins. Proteomic analyses and other experiments demonstrated that, in addition to FAS, the translation of many TCR signaling genes (such as CD69, CD28, PD1, and CTLA4) is also regulated by AMBRA1, suggesting that AMBRA1 has a broad impact on the translation of T cell signaling proteins.
The research paper titled "AMBRA1 controls the translation of immune-specific genes in T lymphocytes" was published online in Proceedings of the National Academy of Sciences of the United States of America (PNAS) on Oct. 21, 2024.
In summary, this study found that the AMBRA1 gene plays a crucial role in the translational regulation of TCR activation and FAS-induced T cell death signals. Researchers’ work provided potential directions for future research in T cell translation and immunotherapies targeting translational regulation.
The research was jointly conducted by Prof. YAO Yikun’s group and Prof. Michael J. Lenardo’s group from the National Institutes of Health. This work was supported by grants from the National Natural Science Foundation of China, Ministry of Science and Technology of China and CAS.
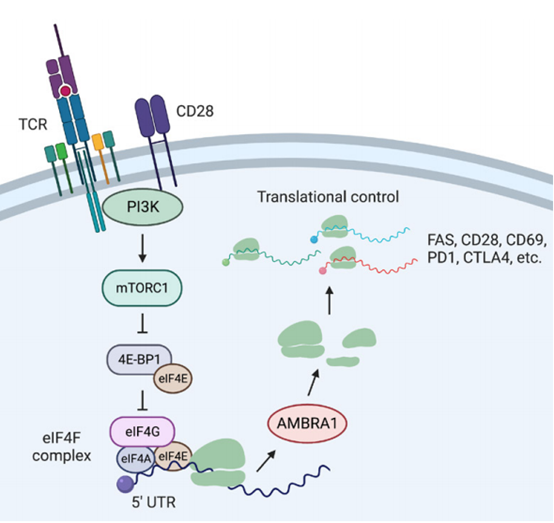
AMBRA1 regulates T cell cycle and T cell death by controlling immune-specific genes’ translation. (Image provided by Prof. YAO Yikun's team)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/
