Researchers Link Aberrant Chromatin Accessibility to Endogenous Fructose Metabolism Reprogramming in Leukemogenesis
A research team led by Dr. WANG Lan from the Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences (CAS), identified a linkage between aberrant epigenetic chromatin accessibility and reprogrammed endogenous fructose metabolism in the regulation of leukemogenesis. Entitled “SMARCA5 reprograms AKR1B1-mediated fructose metabolism to control leukemogenesis”, this study was published online in Developmental Cell on May 21st, 2024.
Acute myeloid leukemia (AML) is an aggressive clonal hematologic malignancy characterized by aberrant self-renewal and immature of hematopoietic stem/progenitor cells (HSPCs). Several studies have revealed genomic and epigenetic alterations of leukemia in regulating gene transcription, chromatin state, and DNA modifications, which are considered to be responsible for the rise of leukemia stem cells (LSCs) and leukemia initiation, progression and relapse. Recent studies have identified a significant variation in chromatin accessibility as a novel feature of AML, while the factors responsible for this alteration remain largely unknown.
Metabolic reprogramming is closely related to tumorigenesis, including leukemogenesis. Previous studies have found that exogenous fructose is essential to promote the development of AML, and since it is known that endogenous fructose can also be generated from glucose via the polyol pathway, the question of whether this endogenous fructose is also essential for leukemogenesis remains elusive.
To systematically identify the factors responsible for the aberrant of chromatin accessibility in AML, a research team led by Dr. WANG Lan performed CRISPR screening in AML cells and found that SMARCA5, an ATPase of the ISWI chromatin remodeling complex, is primarily responsible for the aberrant chromatin accessibility in AML. Through a combined analysis using multiple databases, AML cell lines, conditional knockout mice and samples of AML patients, they found that SMARCA5 is indeed essential for leukemogenesis in vitro and in vivo.
Mechanistically, through combined analysis using RNA-seq, ATAC-seq, CUT&Tag and metabonomics, the researchers found SMARCA5 mediated open chromatin accessibility could promote the expression of AKR1B1, an aldo/keto reductase, by recruiting transcription co-activator DDX5 and transcription factor SP1, and then higher expression of AKR1B1 could reprogram endogenous fructose metabolism to contribute to leukemogenesis. Importantly, treatment of AML with epalrestat, a clinical inhibitor of AKR1B1, has been shown to be effective, which may offer a new therapeutic strategy for leukemia.
Dr. WANG Lan is the corresponding author, and Dr. YU Pengcheng, Dr. HOU Dan, Dr. CHANG Binhe from SINH and Dr. LIU Na from Shanghai Changhai Hospital, Naval Medical University are the co-first authors of the paper.
The work received support from the National Key Research and Development Plan of China, the National Natural Science Foundation of China, the Shanghai "Science and Technology Innovation Action Plan", as well as the technology and animal platform of SINH.
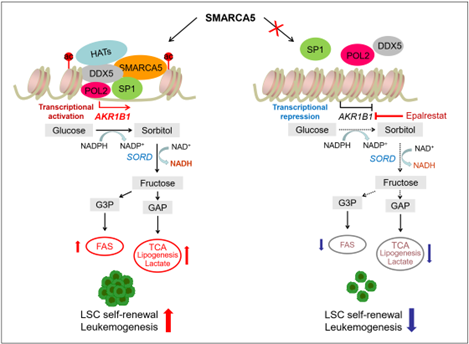
A model depicting how SMARCA5 reprograms AKR1B1-mediated fructose metabolism to control leukemogenesis. (Image by Prof. WANG Lan’s group)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/
