Scientists Uncover Molecular Mechanisms of Liver Regeneration via Kupffer Cell-derived IL-6 Mediated Activation of Progenitor Cells
The liver possesses a remarkable regeneration capacity under injury conditions. Hepatocyte, which accounts for ~70% of total cells within the liver, has been reported to contribute to liver regeneration via reprogramming into liver progenitor-like cells (LPLCs). However, the molecular mechanisms underlying hepatocyte reprogramming remain largely uncharacterized. ScRNA-seq profiles transcriptome at single-cell resolution, making it the most appropriate technology to identify diverse cellular states and to decipher the molecular dynamics underlying state transition.
Utilizing scRNA-seq, researchers identified the subpopulations of hepatocytes upon liver injury, among which a subpopulation exhibiting transcriptomic features of progenitor cells was regarded as LPLCs. By decoding transcriptomic changes across hepatocyte subpopulations, researchers reconstructed the cellular roadmap during LPLC formation, found that immune related pathways were highly correlated with LPLC formation, and identified a set of reprogramming-related genes (RRGs). In vivo experiments based on lineage-specific deletion tools (mice models) demonstrated that liver resident Kupffer cells (KCs) depletion rather than other immune cells (B cell, T cell, natural killer cell, neutrophil, and monocyte-derived macrophage) would lead to a substantial decrease in LPLC formation. To investigate how KCs affect LPLC formation, researchers then performed scRNA-seq and found a set of KC-specific secretion factors, including IL-1a, IL-1b, and IL-6. Further, in vivo screening demonstrated that IL-6 acted as a niche signal repurposed for hepatocyte reprogramming via STAT3 activation. To define the role of STAT3 in reprogramming, chromatin immunoprecipitation sequencing (ChIP-seq) was performed. Activated STAT3 was found to drive RRG expression through binding to their pre-accessible enhancers. Notably, RRGs were activated through injury-specific enhancers rather than liver embryogenesis-related ones. Collectively, these findings depict an injury-specific niche signal and the inflammation-mediated transcription in driving the conversion of hepatocytes into a progenitor phenotype.
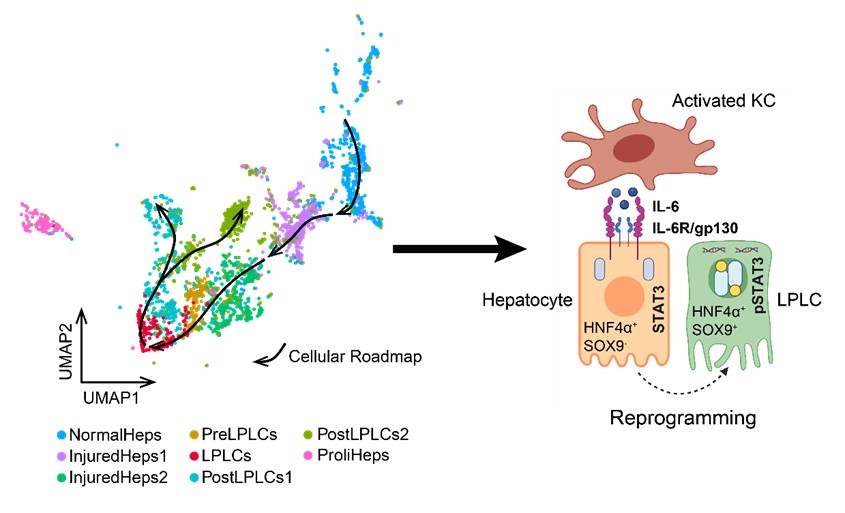
ScRNA-seq delineates the molecular mechanism of hepatocyte reprogramming during liver regeneration. (Image provided by Prof. LI Hong’s group)
This study suggests a concept that proinflammatory signaling may be repurposed to directly induce the transcription of progenitor-related genes for injury-induced cell dedifferentiation in mammals. These findings provide a paradigm to understand reprogramming in other injured tissues.
Prof. LI Hong's group and Prof. LI Yixue's group from SINH performed bioinformatic analysis to provide candidate molecules and Professor HUI LiJian's group from CEMCS performed experiment design and validation.
This work was published online in Cell Stem Cell on Feb. 13th, 2023, as a research article entitled “Kupffer cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers”.
This work was supported by the fundings from Chinese Academy of Sciences, Ministry of Science and Technology, National Natural Science Foundation of China, Shanghai Municipal Science and Technology Commission, and Shanghai Municipal Development & Reform Commission.
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/
