Researchers Jointly Reveal Novel Mechanisms and Therapeutic Targets for Skin Aging
Mammalian skin epithelium, including interfollicular epidermis (IFE) plus hair follicle (HF) and other appendages, undergoes prominent aging changes such as hair loss/graying, epidermal hypotrophy, and reduction of wound healing capability. As a fast turnover tissue, aging of skin epithelium can be attributed to regressive changes in its residing stem cells (SCs). It was shown that DNA damage induced HF stem cell (HFSC) elimination via IFE trans-differentiation is a key mechanism underlying HF aging. This was linked with degradation of COL17A1 collagen on HFSC surface, but cell-intrinsic factors mediating the aging HFSCs' fate transition were unclear. In addition, similar HFSC-to-IFE trans-differentiation also happens during skin wound healing. But it was unclear whether aging and wound response in HFSC share a common molecular mechanism.
Prof. ZHANG Liang's group from the Institute of Nutrition and Health, of the Chinese Academy of Sciences discovered that mouse HFSC aging is initiated by their intrinsic up-regulation of miR-31, a microRNA that can be induced by physical injury or genotoxic stress. Using transgenic and conditional knockout mouse models plus lineage tracing technique, researchers showed that miR-31 acts as a key driver of HFSC aging by directly targeting Clock, a core circadian clock gene whose deregulation activates a MAPK/ERK cascade to induce HFSC depletion via transepidermal elimination. Importantly, researchers found that blocking this pathway by either conditional miR-31 ablation or clinically approved MAPK/ERK inhibitors provided safe and effective protection against skin aging.
Collaborated with Prof. LI Qingfeng's group from the Shanghai Ninth People’s Hospital, researchers further showed that miR-31 up-regulation is linked with not only human HF aging but also human epidermis aging, suggesting that miR-31 underlies both HF and epidermis aging in human.
This study united aging and healing, two seemingly contradictory processes, into a common molecular pathway in HFSCs. It is a double-edge blade that promotes immediate healing at the cost of long-term SC preservation. Importantly, both miR-31 ablation and clinically approved MAPK/ERK inhibitors (e.g. Trametinib) were found to provide effective protection against skin aging without discernible adverse effects, enlightening promising therapeutic avenues for treating skin aging and other genotoxic stress induced skin conditions such as Radiodermatitis.
This study entitled "A stress-induced miR-31–CLOCK–ERK pathway is a key driver and therapeutic target for skin aging" was published online in Nature Aging on Aug. 16th, 2021. Two related patents have been filed to China National Intellectual Property Administration. This research was funded by the China Ministry of Science and Technology, the Chinese Academy of Sciences, the National Natural Science Foundation of China, and the Science and Technology Commission of Shanghai Municipality.
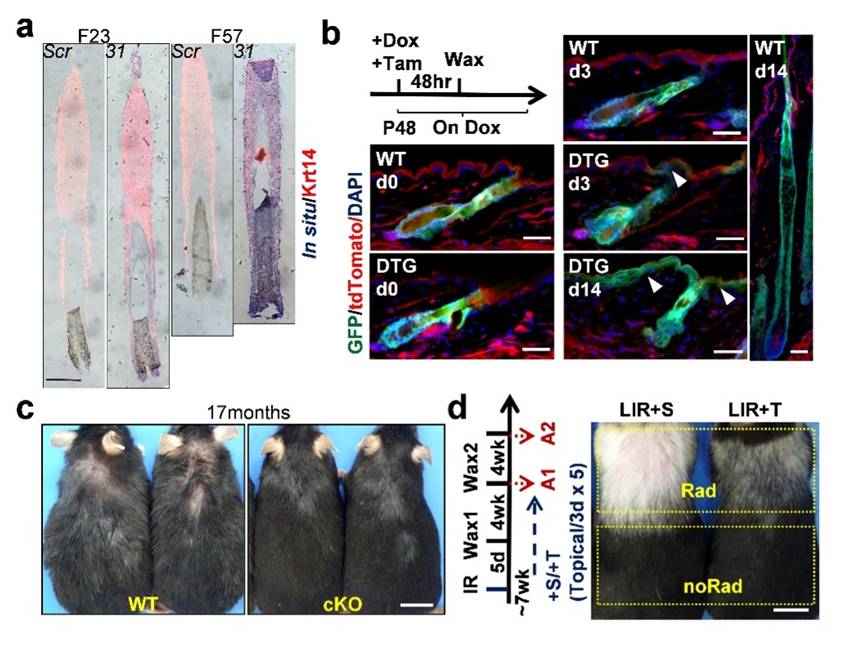
a) In situ analysis showing upregulation of miR-31 in aged human scalp HF. F21 and F57: females at age 21 and 57 respectively. b) Lineage tracing of HFSC progenies (GFP) showing that transgenic miR-31 overexpression drives HFSC to IFE differentiation. c) Aged mice with conditional miR-31 ablation in HFSC (cKO) displayed more youthful looking hair coat. d) Topical application of Trametinib (+T) suppressed IR induced premature skin aging in mice. The control mice were treated with solvent (+S). Rad: IR treated skin region. noRad: untreated skin. (Image provided by Prof. ZHANG Liang's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
Web: http://english.sinh.cas.cn/
