Scientists Discover Novel Oncogenic Driver Gene in Human Lung Cancer
A research team led by Prof. WANG Yuexiang from Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences discovered a novel oncogenic driver gene in human lung cancer. The study entitled “E3 Ligase MKRN3 Is a Tumor Suppressor Regulating PABPC1 Ubiquitination in Non-Small Cell Lung Cancer” was published online in the scientific journal J Exp Med on June 18, 2021.
Lung cancer is the leading cause of cancer-related mortality worldwide, with 1.8 million deaths globally in 2020. Approximately 85% of all lung cancer cases are non-small cell lung cancers (NSCLCs). Although tyrosine kinase inhibitors (TKIs) and immunotherapy have contributed to significant survival benefits in some patients, the overall survival rates for NSCLCs remain low. In particular, patients with NSCLC that are driven by KRAS mutations are often unresponsive to TKIs and have a poor prognosis. Targeted therapy against mutant KRAS-driven tumors (in up to 30% of NSCLCs) has proved challenging. Indeed, although inhibitors for the KRASG12C mutant have approved to treat NSCLC patients, a general strategy that targets all KRAS mutants remains elusive. There is therefore an urgent need to identify new driver genes and develop therapeutic strategies to benefit a broader patient population, especially those with KRAS mutations.
Central precocious puberty (CPP) is largely caused by germline mutations in the MKRN3 gene. Interestingly, CPP has been epidemiologically linked to various diseases in adulthood, including cancers. Cohorts of individuals with CPP show an increased risk of malignancies such as lung cancers. There is also compelling evidence that sex steroid hormones and reproductive factors play a role in the genesis of lung cancer, yet the mechanisms are unclear.
To investigate whether central precocious puberty-associated MKRN3 gene is mutated in human cancers, the research team led by Prof. WANG Yuexiang queried The Cancer Genome Atlas (TCGA) Pan-Cancer genomic data sets. Strikingly, MKRN3 is frequently mutated in NSCLCs. MKRN3 aberrations are significantly enriched in human NSCLC samples harboring oncogenic KRAS mutations.
The researchers further presented genetic, functional, mouse models (genetically engineered mouse model and chemically induced lung cancer model) and mechanistic data that identify the central precocious puberty-associated gene MKRN3 gene as a bona fide tumor suppressor in NSCLC, uncover its tumor suppressing mechanism and highlight MKRN3-PABPC1 axis deregulation as a key pathway in lung cancer oncogenesis. MKRN3 inactivation leads to lung cancer proliferation and progression through PABPC1 ubiquitination mediated global protein synthesis. MKRN3 restoration in MKRN3-inactivated NSCLC suppresses tumor growth in nude mice. Therefore, molecular interventions targeting MKRN3 deficiency may have therapeutic potential for KRAS-mutant NSCLC treatment.
These intriguing findings showed that biological mechanisms of central precocious puberty are relevant in tumorigenesis, and thereby highlighted that recent therapeutic developments for central precocious puberty could also serve as anticancer drugs.
The research was supported by Profs. TANG Hua and ZANG Yuan-Sheng from Changzheng Hospital, Dr. SHEN Yangying from Renji Hospital , Profs. XIAO Yichuan, HUANG Tao, QIN Jun from SINH, Dr. ZHANG Miaoying from Children's Hospital, Fudan University, and Prof. HE Yi from No. 1 Hospital of Jiaxing in Zhejiang Province of China.This project was financially supported by National Natural Science Foundation of China, Ministry of Science and Technology of China, Science and Technology Commission of Shanghai Municipality, and Chinese Academy of Sciences.
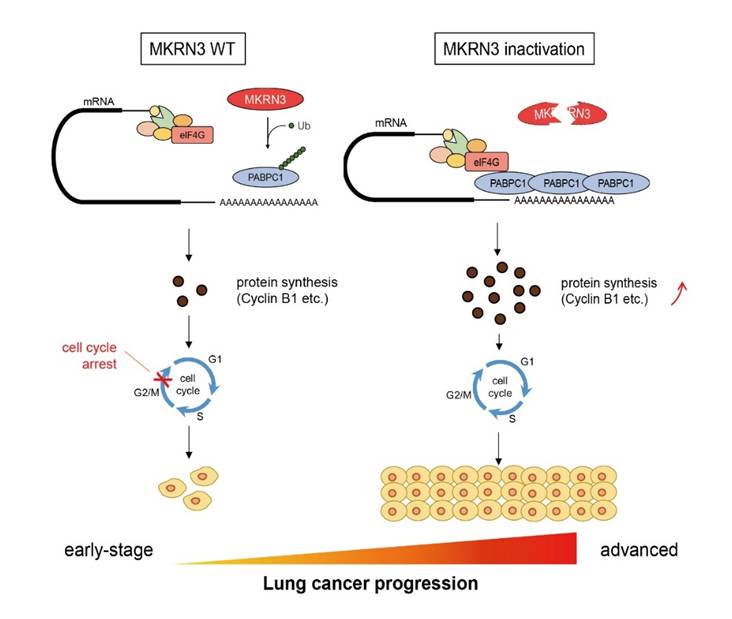
A model depicting how the MKRN3-PABPC1 axis controls cell proliferation and progression in lung cancer. (Provided by Prof. WANG Yuexiang's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
Web: http://english.sinh.cas.cn/
