Loss of p53 in Mesenchymal Stem Cells Promotes Bone Remodeling
The origin of mesoderm is a great event during evolution which establishes bilateral symmetry and makes the formation of various organ and systems possible. In mammals, a special cell population, mesenchymal stem cells (MSCs), exists mostly in mesoderm and neural crest, has been isolated and identified as pluripotent adult stem cells with the capacity of immunoregulation and tissue regeneration.
MSCs are the sole source of osteoblast progenitors. Their differentiation coordinate with the activities of osteoclasts to maintain a balanced bone remodeling. In this context, the transcription factor p53, a well-known tumor suppressor, plays a determinant role as a regulator of osteogenesis and osteoblast differentiation. Notably, tumor bone metastasis is frequently associated with aberrant bone remodeling and it is now evident that cancer cells can selectively silence p53 activities in tumor associated MSCs. However, the pathophysiological fine-tuning of MSCs regulated bone remodeling by p53 remains largely undetermined.
In a recent paper entitled “Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin”, Dr. WANG Ying and SHI Yufang’s group defined the role of p53 in the regulation of MSC differentiation by revealing another layers of complexity.
Researchers show that mice with p53 deletion systemically or specifically in MSCs possess significantly higher bone density. Further mechanistic studies reveal that in MSCs, p53 negatively regulates the transcription of osteoprotegerin (OPG), a key soluble protein capable of inhibiting osteoclast differentiation factor RANKL, and thus tilts the balance of bone remodeling toward osteogenesis. Notably, high expression of Opg or its combination with low level of p53 are prominent features in clinical cancer lesion of osteosarcoma and prostate cancer respectively and correlate with poor survival. Using a mice model of prostate cancer, the researchers find that tumor cells together with androgen can suppress p53 expression and enhance local OPG production, leading to an enhancement of bone density.
These findings support the notion that MSCs represent a cellular source of OPG, which in turn is regulated by the p53 status. This p53-OPG axis exists in physiological bone remodeling and cancer bone metastasis.
The research was published online in Cell Death & Differentiation on July 22, 2020. It was a collaborative study by the Shanghai Institute of Nutrition and Health of the Chinese Academy of Sciences, the First Affiliated Hospital of Soochow University, and University of Rome Tor Vergata.
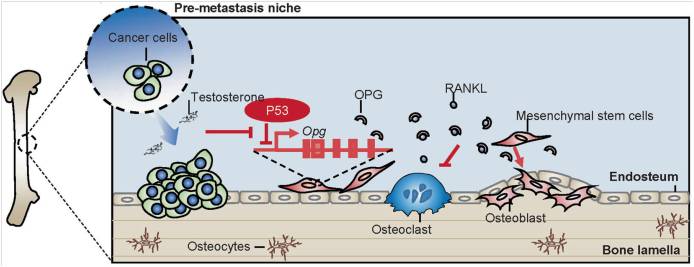
Schematic representation of the proposed molecular mechanism by which p53 regulates bone remodeling. (Image provided by Dr. WANG Ying and Dr. SHI Yufang’s group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
