Researchers Reveal Role of SETD2 in MDS-associated Leukemogenesis
A recent study, published online in Blood, revealed the role and mechanism of SETD2 in MDS-associated leukemogenesis. It was performed by Prof. WANG Lan’s group from Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences. WANG’s lab identified that SETD2 deficiency predicts poor prognosis in MDS and accelerated MDS-associated leukemogenesis via S100a9.
Myelodysplastic Syndromes (MDS) is one of the most common myeloid malignancies characterized by bone marrow (BM) dysplasia, inefficient hematopoiesis, cytopenia and a risk of progression to acute myeloid leukemia (AML). SETD2, the histone H3 lysine 36 methyltransferase, plays an important role in the pathogenesis of hematologic malignancies. A lot of SETD2 mutations have been found in chronic lymphocytic leukemia (CLL), MLL-NRIP3 and MLL-AF9-driven acute leukemia. However, the role of SETD2 in MDS has been unclear.
In the study, researchers found that low expression of SETD2 correlated with shortened survival in MDS patients. Loss of Setd2 enhanced the ability of NHD13+ hematopoietic stem progenitor cells (HSPCs) to self-renew, with the impairment in differentiation/cell death and the increase in the symmetric self-renewal division. Early progression of MDS to AML in the Setd2-deleted NHD13 mice also associated with abnormal erythroid differentiation and reduced cell death. Setd2 deletion increased the symmetric self-renewal division of the NHD13-expressing HSCs, which might also account for the higher risk for the development of leukemia in the NHD13/Setd2Δ/Δ mice. Loss of Setd2 promoted the maintenance of the MDS-derived leukemia, and the growth of MDS-associated leukemia cells was inhibited by increasing H3K36me3 level.
Furthermore, Setd2 deficiency upregulated hematopoietic stem cell (HSC) signaling and downregulated the myeloid cell differentiation pathway in the NHD13+ HSPCs. RNA-seq and ChIP-seq analysis indicated that S100a9, S100 calcium binding protein A9, was a target gene of Setd2, and the addition of recombinant S100a9 protein weakened the effect of Setd2 deficiency on the NHD13+ HSPCs. In contrast, downregulation of S100a9 led to the decreases of its downstream targets, including 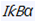 and Jnk, which influence the self-renewal and differentiation of HSPCs.
and Jnk, which influence the self-renewal and differentiation of HSPCs.
This study revealed, for the first time, that Setd2 acts as an important tumor suppressor in the transformation of NHD13-driven MDS to AML, providing therapeutic targets for treatment of MDS in clinical.
This work entitled “SETD2 deficiency predicts poor prognosis in MDS and accelerated MDS-associated leukemogenesis via S100a9” was published online in Blood on Mar 20, 2020, and was supported by National Natural Science Foundation of China, Ministry of Science and Technology, Chinese Academy of Sciences and State Key Laboratory of Medical Genomics.
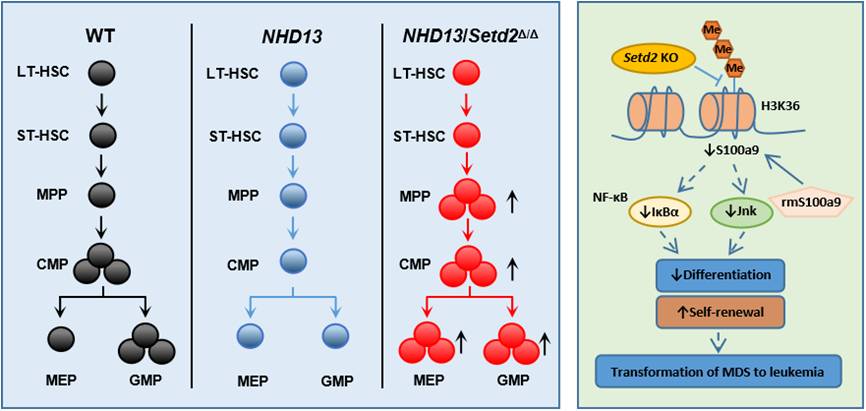
Setd2 plays an important role in the transformation of NHD13 driven MDS to AML.
(Image provided by Prof. WANG Lan's team)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
