Paracrine AREG from Treatment-generated Senescent Stromal Cells Elicits Drug Resistance of Cancer Cells and Creates an Immunosuppressive Microenvironment
Researchers at Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences discovered that amphiregulin (AREG), a soluble factor produced by the treatment-damaged tumor microenvironment (TME), confers remarkable resistance on surviving cancer cells and simultaneously creates an immunosuppressive niche by engaging PD-L1, a type I transmembrane protein that potently activates the immune checkpoint.
Cellular senescence was originally discovered in vitro in 1960s and was postulated to function as a tumor suppressive mechanism operative in incipient cancer cells. However, increasing lines of evidence showed that senescent cells could indeed drive tumorigenesis instead of limiting it. The tumor-promoting and tumor suppressing functions of cellular senescence seemed to be incompatible, but later on it was disclosed that most senescent cells secrete numerous pro-inflammatory, growth-stimulatory and extracellular matrix-modifying factors, collectively referred to as the senescence-associated secretory phenotype (SASP), which can substantially impact on tumorigenesis.
Early investigation into the effect of SASP factors on the immune system revealed that certain factors elicit the clearance of senescent cells, raising the possibility that the SASP ensures that senescent, pre-neoplastic cells are removed, thus preserving tissue homeostasis.
However, researchers from Dr. SUN Yu's group at SINH recently found that once AREG is released from senescent stromal cells into the extracellular space, it enters the circulating blood of post-treatment cancer patients and can be exploited as a novel and noninvasive biomarker for evaluation of therapeutic effects and clinical prognosis of patient outcome in clinical oncology.
The researchers established AREG as one of the major effectors of the full SASP spectrum, as elimination of AREG from stromal cells caused markedly weakened cancer cell malignancies, including proliferation, migration, invasion, and more importantly, chemoresistance. They further demonstrated that chemotherapy combined with an AREG-targeting monoclonal antibody achieved an efficacy that was even higher than that generated by chemotherapy plus cetuximab, a FDA‐approved anti-EGFR agent, suggesting that EGFR may not be the sole cell surface receptor interacted by paracrine AREG in the TME.
Researchers explored the feasibility of targeting this response by using therapeutic antibodies that specifically recognize either PD-L1 or its receptor, PD-1, agents that block the immune checkpoint. Preclinical studies showed that such an immune checkpoint blockade (ICB) treatment was more effective when synergizing with conventional chemotherapy, which causes irreparable TME damage and induces substantial AREG expression in stromal compartments, resulting in the formation of an immunosuppressive niche via PD-L1 upregulation in cancer cells.
Surprisingly, in contrast to the ICB agents approved for clinical purposes, AREG mAb exhibited even higher therapeutic efficiency when combined with “classic” chemotherapeutics, a case observed in both prostate cancer and breast cancer animal models humanized for ICB studies.
Understanding the immunomodulatory effects of conventional anticancer drugs may have a profound effect on the design of novel and optimal treatment options that allow these agents to be combined with immunotherapies. Successful harnessing of the currently approved anticancer drugs will permit to design more efficient and safer combinatorial therapies, and such a pragmatic strategy is believed to be able to present the best imaginable service to cancer patients, such as turning those immunologically "cold tumors" into "hot foci" through administration of anti-AREG or even anti-SASP agents in future clinics.
The research paper entitled “Targeting amphiregulin (AREG) derived from senescent stromal cells diminishes cancer resistance and averts programmed cell death 1 ligand (PD‐L1)‐mediated immunosuppression” was published online in Aging Cell on September 9, 2019. This work was supported by the Ministry of Science and Technology of China, National Natural Science Foundation of China and the Chinese Academy of Sciences, with the technical assistance from the research platforms of SINH.
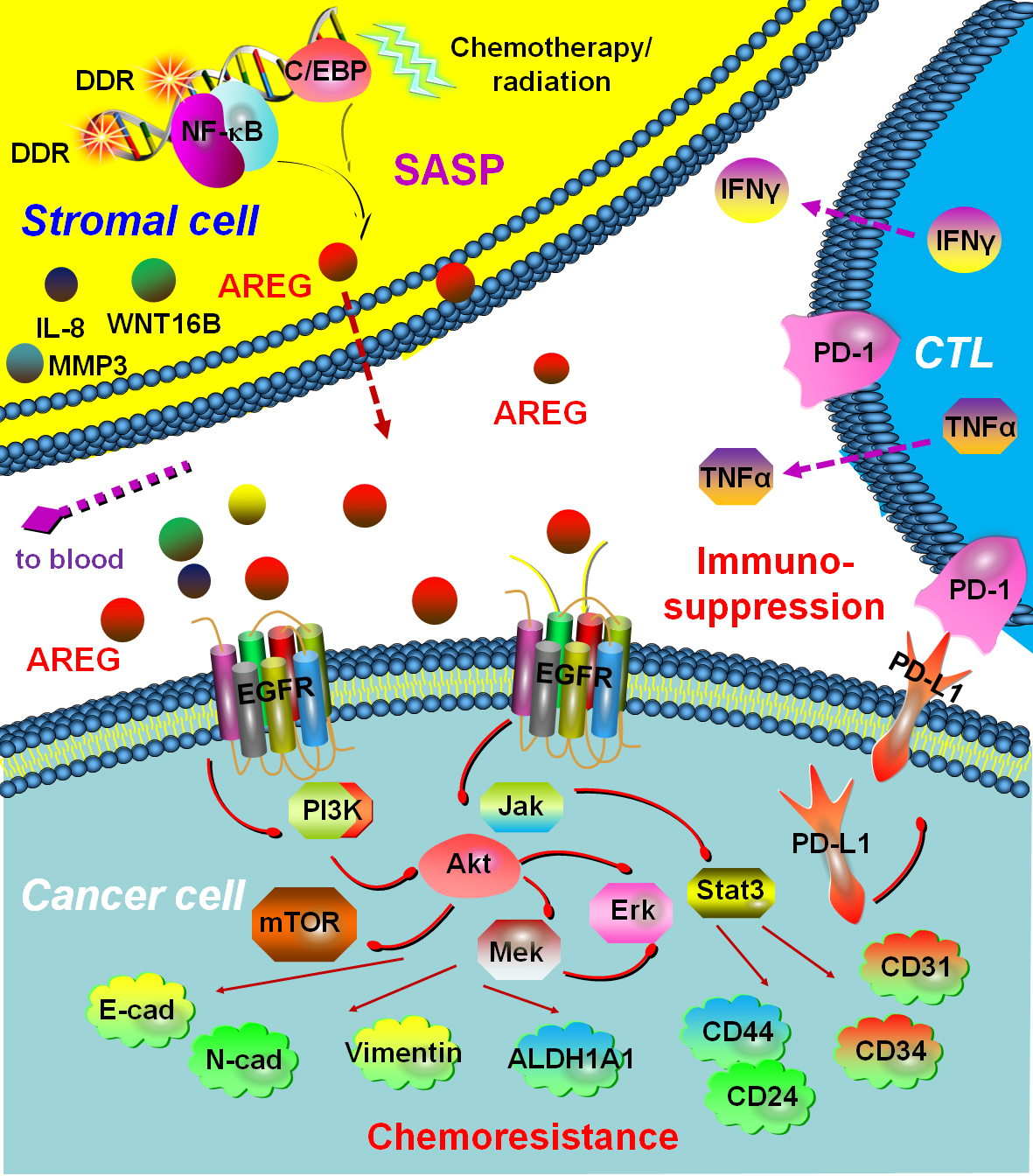
Paracrine AREG from treatment-generated senescent stromal cells elicits drug resistance of cancer cells and creates an immunosuppressive microenvironment. (Image by Dr. Sun's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
