Activation of DR3 Signaling Exacerbates Intestinal Inflammation Through Regulating ILC3s
TNF-like ligand 1A (TL1A) and death receptor 3 (DR3) are a ligand-receptor pair that belongs to the TNF superfamily and play important roles in Inflammatory Bowel Disease (IBD). DR3 has been found to be expressed by various immune cells and regulate immune responses in dual directions. DR3 signaling has been reported to play a pathogenic role in IBD through promoting Th1 and Th17 response. On the other hand, it was reported that DR3 signaling is indispensible for the maintenance of intestinal Tregs during colitis which suppress the exacerbation of colitis. Group 3 innate lymphoid cells (ILC3s) express high level of DR3 and play crucial roles in intestinal immunity. However, the effect of TL1A/DR3 signaling on ILC3s remains elusive.
Recently, a research team led by Dr. QIU Ju from Shanghai Institute of Nutrition and Health of Chinese Academy of Sciences found that activation of DR3 signaling plays a pathogenic role in colitis by regulating ILC3s. The study entitled "Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation" has been published online on July 30th in Nature Communications.
The research team has reported that DR3 signaling exacerbates intestinal inflammation through ILC3s, yet finally “expulses” ILC3s from the intestine. Mechanism studies showed that activation of DR3 signaling promotes GM-CSF expression from ILC3s through p38 signaling pathway. GM-CSF leads to accumulation of eosinophils, neutrophils and CD11b+CD11c+ myeloid cells in the large intestinal lamina propria, which induce loss of ILC3 through an IL-23-dependent manner and exacerbates colitis. Neutralization of TL1A by soluble DR3 ameliorates both DSS and anti-CD40 antibody-induced colitis. Moreover, ILC3s are required for the deleterious effect of anti-DR3 antibodies on innate colitis.
The study helps to elucidate the pathogenicity of TL1A, which has been reported to be increased in inflamed tissue of patients with IBD. Final reduction of ILC3s can result in contraction of the inflammation, which works as an efficient feedback mechanism to control overt inflammatory responses. In addition, loss of ILC3s through GM-CSF/IL-23 axis proved by this research provides an important mechanism for decreased proportion of IL22+ILC3s in patients with Crohn’s disease.
LI Jingyu and SHI Wenli are co-first authors of this work. This study was accomplished with the great help from Dr. SHENG Huiming and his research team from Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Dr. GUO Xiaohuan from Tsinghua University and Dr. ZHOU Liang from University of Florida. This work was supported by grants from National Natural Science Foundation of China, Ministry of Science and Technology, and Chinese Academy of Sciences.
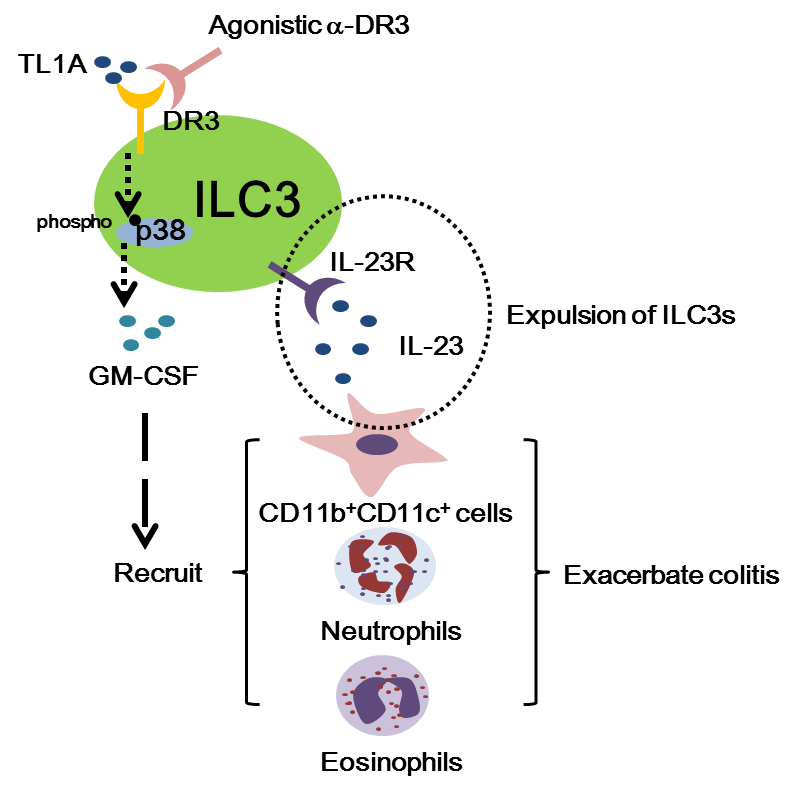
Schematic illustration of the intestinal immunity regulated by activation of DR3 signaling on ILC3s. (Image by Dr. QIU Ju's team)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
