Researchers Reveal Role of Autophagy in Glucocorticoid-Induced BAT Whitening
Adipose tissue is the primary organ of storing energy, which includes white, brown and beige adipocytes. White fat could convert into brown fat-like containing UCP1-positive cells (beige adipocyte) under external stimulation such as cold, a process known as white fat browning.
Extensive studies have been carried out and significant progress has been made on the understanding of white fat browning over the past decades. Researchers found that brown adipose tissue (BAT) acquires white adipose tissue (WAT) cell features defined as BAT whitening under certain circumstances. The nature of BAT whitening and the mechanisms involved in this regulation, however, remain poorly understood.
Recently, a study led by Prof. GUO Feifan from Shanghai Institute of Nutrition and Health (SINH), Chinese Academy of Sciences (CAS) revealed that autophagy inhibition prevents glucocorticoid-increased adiposity via suppressing BAT whitening.
Glucocorticoids (GCs) are a class of steroid hormones and exert their effects by binding to the nuclear receptor glucocorticoid receptor. GC and their synthetic analogs, such as dexamethasone (Dex), are widely used to treat various diseases. Unfortunately, the development of side effects limits the long-term use of GCs. One of such effects is the increased adiposity in WAT. Understanding of the mechanisms underlying GC-increased adiposity will help to develop more effective clinical application.
In this study, researchers showed that fat mass content and WAT weight were increased in mice treated with Dex for one week. Meanwhile, researchers found that one-week Dex treatment induced BAT whitening, characterized by the accumulation of enlarged lipid droplets and increased lipid contents. In addition, UCP1 expression and oxygen consumption rate were decreased in BAT cells of Dex-treated mice. Furthermore, ATG7 (autophagy related 7) expression and autophagy was induced in BAT by Dex. Autophagy plays a wide variety of physiological and pathophysiological roles. It is a cellular process that degrades intracellular organelles and proteins via autophagy-related genes. Treatment with the autophagy inhibitor chloroquine or adenovirus-mediated ATG7 knockdown prevented Dex-induced BAT whitening and fat mass gain. Moreover, Dex-increased ATG7 expression and autophagy was mediated by BTG1 (B cell translocation gene 1, anti-proliferative)/CREB1 (cAMP response element binding protein 1) signaling. The importance of BTG1 in this regulation was further demonstrated by the observed BAT whitening in adipocyte-specific BTG1- overexpressing mice. BTG1 knockdown in BAT attenuated Dex-induced BAT whitening and fat mass gain.
Taken together, this work showed that Dex induces a significant whitening of BAT via BTG1- and ATG7-dependent autophagy, which might contribute to Dex-increased adiposity. These results provide new insights into the mechanisms underlying GC-increased adiposity and possible strategy for preventing GC-induced side effects via the combined use of an autophagy inhibitor.
The work entitled “Autophagy inhibition prevents glucocorticoid-increased adiposity via suppressing BAT whitening” (https://doi.org/10.1080/15548627.2019.1628537) was published online in Autophagy on June 11th, 2019. Dr. DENG Jiali, a postdoctoral fellow at SINH, is the first author of the research paper. Prof. GUO Feifan is the principal corresponding author as the lead contact and Associate Professor XIAO Fei is the co-corresponding author for this work.
The research was supported by Prof. JI Hongbin at Shanghai Institute of Biochemistry and Cell Biology of CAS, Prof. HU Guohong, Prof. YING Hao, Prof. CHEN Yan and Prof. ZHAI Qiwei at SINH. This research was funded by grants from the National Natural Science Foundation of China, Basic Research of Shanghai Science and Technology Commission and CAS.
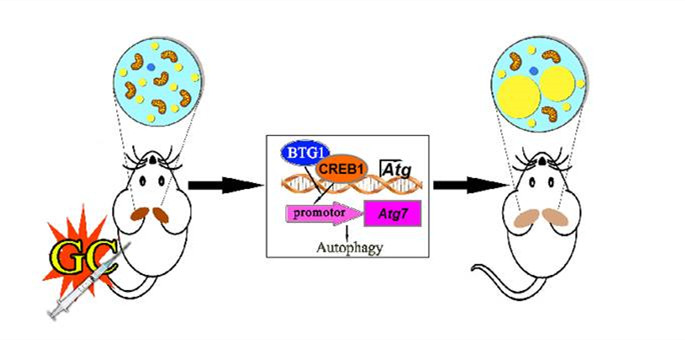
The model of glucocorticoid-induced BAT whitening via autophagy.
(Image provided by Prof. Guo Feifan’s group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
