Researchers Reveal New Function of Amino Acid Sensing Protein ATF4 in Regulation of Inflammatory Bowel Disease
A recent study published in Gastroenterology revealed the new function of amino acid sensing protein activating transcription factor 4 (ATF4) in maintaining the secretory function of Paneth cell and regulation of intestinal inflammation.
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is one of the most prevalent gastrointestinal diseases worldwide. To date, more than 200 IBD risk loci have been identified to be related to the inflammatory response, endoplasmic reticulum (ER) stress, innate and adaptive immunity, antimicrobial defence, autophagy, and gut mircobiota.
Paneth cells are specialized epithelial cells located at the base of small intestinal crypts that act as important effectors of innate immunity through their secretion of antimicrobial peptides. Recent works have reported that the level of amino acids is closely related to the secretion of antimicrobial peptides by Paneth cells, and disruption of genes associated with amino acid homeostasis may increase the susceptibility to IBD.
Prof. Guo Feifan's group at the Shanghai Institute of Nutrition and Health (SINH) of Chinese Academy of Sciences (CAS) has been engaged in the study of the mechanism of amino acid sensing for years. In previous studies, researchers found that amino acid sensing protein ATF4 plays an important role in regulating glucose and lipid metabolism in the central and liver, but its role in the pathogenesis of IBD remain poorly understood.
In the recent published study, assistant research fellow HU Xiaoming, supervised by Prof. GUO Feifan and Prof. LIU Zhanju at the Shanghai Tenth People’s Hospital of Tongji University demonstrated that levels of ATF4 were significantly decreased in inflamed intestinal mucosa from patients with active CD or active UC, compared with uninflamed regions or intestinal mucosa from healthy controls. ATF4 expression was also down-regulated in colonic epithelia from mice with colitis compared to control.
Furthermore, researchers found that intestinal epithelial cell (IEC)-specific Atf4 knockout (Atf4ΔIEC) mice developed spontaneous enterocolitis and increased susceptibility to dextran sulfate sodium (DSS)-induced colitis. Moreover, researchers also found that Atf4ΔIEC mice had decreased serum levels of glutamine and reduced levels of antimicrobial peptides, and then alterations in ileal microbiomes. Injections of antimicrobial peptide (recombinant DEFA1) and glutamine reduced the severity of spontaneous enteritis and DSS-induced colitis in Atf4ΔIEC mice. Mechanically, the expression of solute carrier family 1 member 5 (SLC1A5), a glutamine transporter, was directly regulated by ATF4 in vivo and in vitro. Overexpression of SLC1A5 in vitro increased glutamine uptake and expression of antimicrobial peptides, and reduced Atf4-knockdown induced cytokine expression. Moreover, SLC1A5 expression was decreased in inflamed intestinal mucosa of IBD patients and positively correlated with ATF4 expression.
In a word, this work reveals that ATF4 deletion in inflamed intestinal mucosa in IBD decreases glutamine uptake and antimicrobial peptide expression by diminishing Slc1a5 transcription. ATF4 may serve as a therapeutic target for the treatment of IBD.
The work entitled “ATF4 Deficiency Promotes Intestinal Inflammation in Mice by Reducing Uptake of Glutamine and Expression of Antimicrobial Peptides” was published online in Gastroenterology on March 19th, 2019.
The research was supported by Prof. CHEN Yan, Prof. ZHAI Qiwei, and Prof. YING Hao at SINH. Funding support comes from the National Natural Science Foundation of China, Basic Research Project of Shanghai Science and Technology Commission, and CAS.
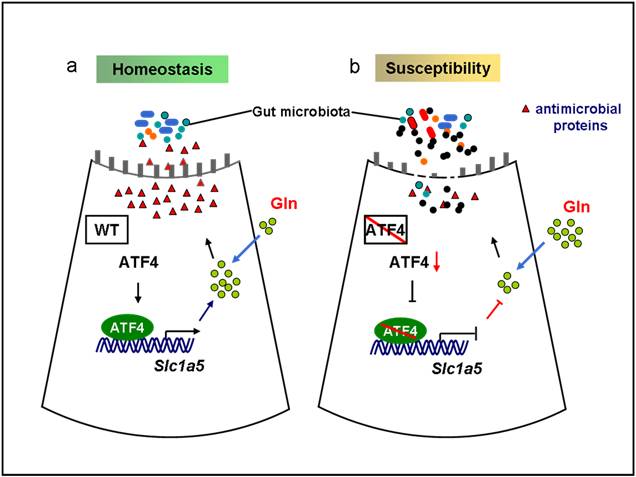
The model of ATF4 in regulating intestinal inflammation.
(Image by Prof. GUO Feifan’s Group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
