Scientists Identify a Novel Mechanism of Post-translational Regulation of Hepatic Lipogenesis via Insulin-Induced Gene
Insulin-induced gene (Insig) is a key regulator for the negative regulation of SREBP-1c-mediated de novo fatty acid synthesis in the liver. However, the upstream signaling that mediates the post-translational regulation of Insig is poorly understood.
Based on the recent findings that a novel metabolic regulator CREBZF inhibits Insig at the transcriptional level and contributes to enhanced hepatic lipogenesis in response to insulin (Zhang F, et al, Hepatology, 2018) and that AMP-activated protein kinase (AMPK)-mediated phosphorylation of SREBP-1c in the regulation of hepatic lipogenesis (Li, Y, et al, Cell Metabolism, 2011), a team of scientists led by Professor LI Yu from Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences demonstrates that AMPK mediates phosphorylation of Insig. AMPK phosphorylates Insig and represses its ubiquitination and degradation via inhibiting the interaction between Insig and the ubiquitin ligase gp78, which prevents the proteolytic processing and activation of SREBP-1c. The beneficial effects of metformin on hepatic steatosis are partially mediated by Insig.
In the study, scientists demonstrated that AMPK interacts with and phosphorylates Insig. Thr222 phosphorylation of Insig-1 by AMPK is required for the protein stabilization of Insig-1 and inhibition of cleavage and processing of SREBP-1 and lipogenic gene expression in response to metformin or A769662. AMPK phosphorylation of Insig ablates its interaction with E3 ubiquitin ligase gp78 and represses its ubiquitination and degradation, whereas AMPK deficiency has the opposite effect showing reduced Insig activity. Hepatic activation of AMPK by metformin is sufficient to reverse gp78-mediated reduction of Insig expression, represses SREBP-1 target gene expression, and protects against hepatic steatosis in diet-induced insulin resistant mice. Moreover, hepatic overexpression of Insig-1 rescues hepatic steatosis in liver-specific AMPKα2 knockout mice fed with HFHS diet. These findings delineate the mechanism of post-translational regulation of Insig, and uncover a novel effector of AMPK that mediates its role in regulating lipid metabolism. Targeting Insig may have the therapeutic potential for treating fatty liver disease and related metabolic disorders.
These findings indicate that CREBZF- and AMPK-mediated transcriptional and post-translational regulation of Insig plays essential roles in the regulation of lipid metabolism in the liver. The dynamic regulation of cellular Insig activity provides a finely tuned mechanism for the cellular regulation of hepatic lipid homeostasis. These studies may provide therapeutic strategies for treating nonalcoholic fatty liver disease, insulin resistance and type 2 diabetes.
This work was published online in Nature Communications on February 7th, 2019 as a research article entitled “Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene”. This study was funded by the grants from Ministry of Science and Technology of China, National Natural Science Foundation of China, Chinese Academy of Sciences and K. C. Wong Education Foundation.
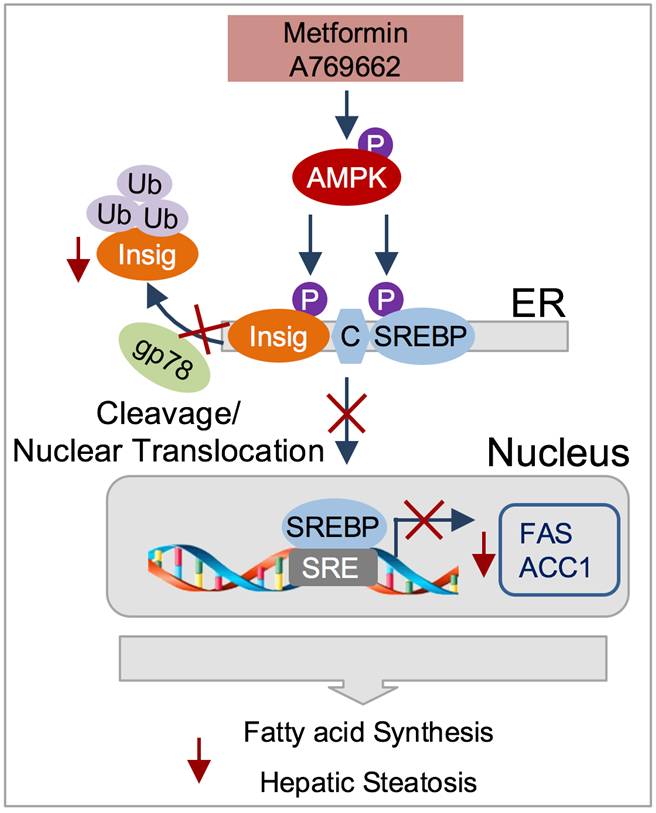
The proposed model for the post-translational regulation of Insig by AMPK Phosphorylation: targeting Insig may have the therapeutic potential for treating fatty liver disease and related metabolic disorders. (Image by Dr. LI Yu’s lab).
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
