Researchers Discovered E Protein Family Member E2-2 Negatively Regulated Leukemogenesis Through AETFC Complex
Oncogenic fusion protein AML1-ETO, generated by the t(8;21) chromosomal translocation, plays an important role in the pathogenesis of t(8;21) leukemia. AML1-ETO exerts its function in a stable protein complex named AETFC (AML1-ETO-containing transcription factor complex). The members of AETFC complex, HEB and E2A belong to E protein family, and they are both vital to AML1-ETO induced leukemogenesis. Interestingly, researchers found that E2-2, the third member of E protein family, was absent in t(8;21) leukemia cells, which suggested that E2-2 could be a negative regulator of t(8;21) leukemogenesis. Thus, the study on how E2-2 regulates AML1-ETO–driven leukemogenesis would provide new insights into the mechanism of t(8;21) leukemogenesis.
A recent study cooperated by researchers from Shanghai Institute of Nutrition and Health, CAS, Shanghai Jiao Tong University School of Medicine and The Rockefeller University found that E2-2 abrogates the function of AETFC complex and negatively regulates leukemogenesis. The underlying mechanism involves E2-2–mediated redistribution of AETFC complex to specific target genes, inducing a dendritic differentiation of the leukemic cells.
In the study, scientisits revealed that the overexpression of E2-2 selectively inhibited the proliferation of AML1-ETO–positive leukemic cells, which requires the bHLH (basis helix-loop-helix) DNA-binding domain. RNA-seq and ChIP-seq analyses indicated that the three E proteins differentially regulate many target genes. In particular, the results show that E2-2 redistributes AETFC to, and activates, some genes associated with dendritic cell differentiation. In AML (acute myeloid leukemia) patients, the expression of E2-2 is relatively lower in the AML1-ETO–positive subtype, and an E2-2 target gene, THPO, is identified as a potential predictor of relapse. Moreover, researchers found that inhibition of E2-2 accelerated leukemogenesis in a mouse model of human t(8;21) leukemia.
Taken together, the three E proteins define a heterogeneity of AETFC complex, which improves the understanding of the precise mechanism. This research also provides a promising potential for an efficient dendritic differentiation of t(8;21) leukemia and for development of the immunotherapy for AML1-ETO-associated leukemia.
The results entitled “Different roles of E proteins in t(8;21) leukemia: E2-2 compromises the function of AETFC and negatively regulates leukemogenesis” was published online in Proceedings of the National Academy of Sciences on December 28th, 2018. This work was completed by research teams led by Dr. SUN Xiaojian from Shanghai Institute of Hematology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Dr. WANG Lan from Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences collaborated with Dr. Robert G. ROEDER from The Rockefeller University. The research work was supported by Prof. CHEN Saijuan, Prof. CHEN Zhu from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Dr. Stephen NIMER from University of Miami, Dr. SHEN Shuhong from Shanghai Children’s Medical Center and Prof. LAN Fei from Shanghai Medical College, Fudan University. The study was funded by the National Key Research and Development Plan of China, National Natural Science Foundation of China, and Chinese Academy of Sciences, etc.
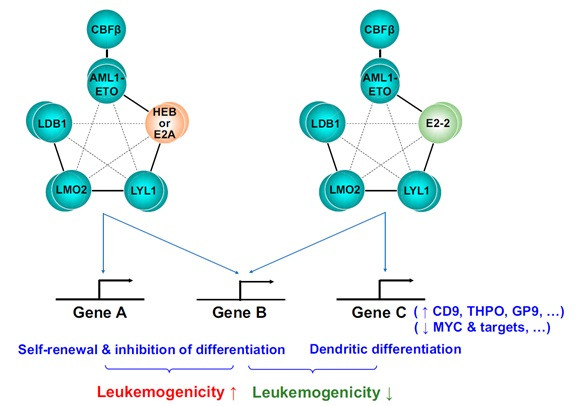
A working model for the different roles of E proteins in AML1-ETO–associated leukemia.
(Image by Dr. WANG Lan's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
