Researchers Discover Novel Mechanisms of a Key ER Chaperone in the Negative Feedback Regulation of Cellular Stress
The ER-associated degradation (ERAD) pathway is activated by the unfolded protein response (UPR) and is vital in removing overloaded proteins to ameliorate ER stress. Autophagy is a noncanonical ERAD pathway degrading cellular contents in the lysosome and has been reported to be triggered upon ER stress to eliminate excess proteins and maintain protein homeostasis. However, the signaling pathways that couple ER stress to autophagy are not completely understood.
Based on the previous findings that berberine relieves hepatic steatosis and maintains energy balance by activating autophagy and FGF21 signaling, a team of researchers led by Professor LI Yu from Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences discoverd that a quality-control ER chaperon, Calreticulin, is induced under ER stress and triggers autophagy via interacting with MAP1LC3 (LC3). This axis promotes the degradation of aberrantly folded proteins and alleviation of ER stress via a negative feedback loop.
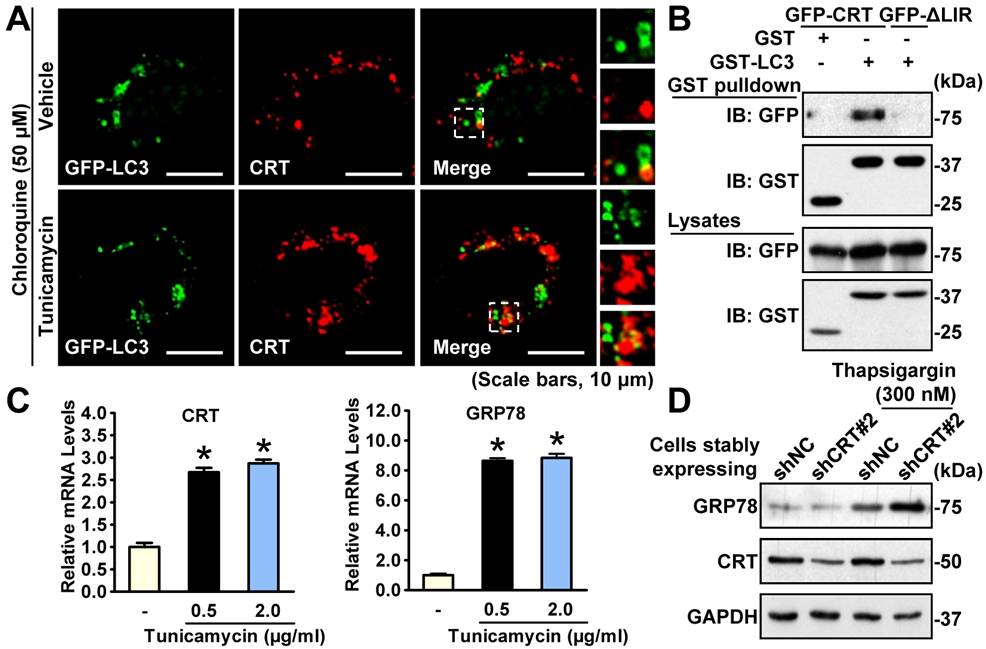
In the study, researchers confirmed the upregulation of Calreticulin under ER stress both in vivo and in vitro. They demonstrated the ER stress-ameliorating role of Calreticulin through genetic overexpression and shRNA-mediated stably knockdown experiments. To study the underlying mechanisms, researchers examined the levels of autophagy in the above contexts and verified the role of Calreticulin in promoting autophagy.
Furthermore, researchers characterized the interaction between Calreticulin and the autophagic protein LC3 and identified its interaction region. Notably, in vitro experiments indicated that this association is indispensable for the beneficial role of Calreticulin in alleviating ER stress via autophagy. All these findings may provide a potential therapeutic strategy for hyperactive ER stress and its related disorders.
This work was published online in Journal of Biological Chemistry on November 14, 2018, (doi:10.1074/jbc.RA118.005166) as a research article entitled "The ER-localized Ca2+-binding protein calreticulin couples ER stress to autophagy by associating with microtubule-associated protein 1A/1B light chain 3". This study was funded by the grants from Ministry of Science and Technology of China, National Natural Science Foundation of China, Chinese Academy of Sciences (CAS), and K. C. Wong Education Foundation.
Calreticulin-autophagy axis in the negative feedback regulation of ER stress. Calreticulin is induced by UPR in response to ER stress. Calreticulin enhances autophagic flux to attenuate cellular stress likely through the alleviation of aberrantly folded proteins. Therapeutic approaches to activate Calreticulin-autophagy signaling may have the potential for treating ER stress and its related disorders.
(Image by Dr. LI Yu’s lab)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn
