IP3R-mediated Ca2+ Signals Govern Hematopoietic and Cardiac Divergence of Flk1+ Cells via Calcineurin-NFATc3-Etv2 Pathways
Ca2+ signals participate in various cellular processes with spatial and temporal dynamics. However, their roles and downstream regulatory pathways in cell fate decisions during early development are not fully understood. Inositol 1,4,5-trisphosphate receptors (IP3Rs)-mediated Ca2+ signals released from the important intracellular Ca2+ store endoplasmic reticulum are critical for early development, but the precise roles and underlying mechanisms of IP3Rs in cell fate decisions remain largely unknown. The pattern of lineage specification of embryonic stem cells (ESCs) and the underlining molecular events faithfully recapitulate the early development of the embryos. Hematopoietic and cardiac lineages are both derived from Flk1+ mesoderm cells, while it remains unclear how Flk1+ progenitor cells determine the alternative cell fate towards one of these lineages.
Recently, a research team led by Dr. YANG Huangtian from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences found the previously unrecognized governing role of IP3Rs in hematopoietic and cardiac fate divergence of mouse embryonic stem cells (mESCs) via IP3Rs-Ca2+-calcineurin-NFATc3-Etv2 pathway.
Deletion of IP3Rs (IP3Rs-tKO) reduced Flk1+/PDGFRa- hematopoietic mesoderm, c-Kit+/CD41+ hematopoietic progenitor cell populations and the colony-forming units activity, while it increased cardiac progenitor cells as well as cardiomyocytes. Concomitantly, the expression of a key regulator of hematopoiesis Etv2 was reduced in IP3R-tKO cells but rescued by the activation of Ca2+ signals and calcineurin or overexpression of constitutively active form of NFATc3. Furthermore, Etv2 was specifically targeted by NFATc3 via its evolutionarily conserved cis-element in differentiating ESCs, which was impaired by IP3R-tKO. In addition, the activation of Ca2+-calcineurin-NFAT pathway reversed the phenotype of IP3R-tKO cells.
These findings provide the new insight into the important role of IP3R-mediated Ca2+ signals in the specific lineage fate decision during early ESC differentiation and the knowledge of how IP3R-mediated Ca2+ directs the selection of mesoderm-derivatives.
The study was collaborated with Professor OUYANG Kunfu from Peking University Shenzhen Graduate School and Professor GAO Shaorong from Tongji University. Professor CHEN Ju from University of California, San Diego provided Itpr1fl/fl2fl/fl3fl/f mice.
The article entitled “IP3R-mediated Ca2+ signals govern hematopoietic and cardiac divergence of Flk1+ cells via the calcineurin-NFATc3-Etv2 pathway” was published online in J Mol Cell Biol on 2017 Apr 13. doi: 10.1093/jmcb/mjx014.
The study was funded by National Natural Science Foundation of China, Ministry of Science and Technology of China and Chinese Academy of Sciences, etc.
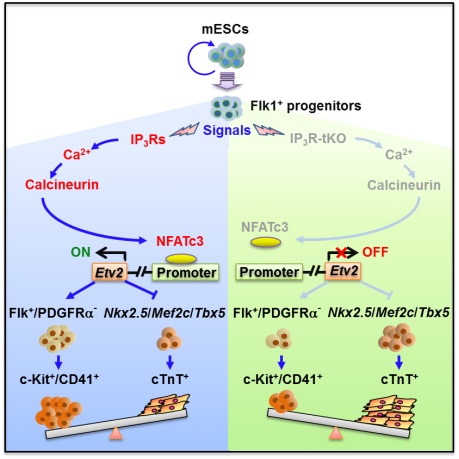
A proposed model for the role of IP3Rs-mediated Ca2+ signals in hematopoietic and cardiac fate commitment of mESCs.
(Image provided by Dr. YANG Huang-Tian's group.)
Correspondence:
Dr. YANG Huang-Tian
Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
320 Yueyang Road, Shanghai 200031, China
E-mail: htyang@sibs.ac.cn
