Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells Improve Cardiac Function without Remuscularization in Infarcted Nonhuman Primates
Myocardial infarction (MI) is the leading cause of morbidity and mortality worldwide. Cell-based therapies are being explored as a therapeutic option for patients with MI. Human pluripotent stem cells (hPSCs), which include both human-embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), are promising seed cells for regenerative medicine as they can be induced to produce theoretically unlimited quantities of progenitor and tissue cells from three germ layers. Recently, the first clinic trial of hESC-derived cardiovascular progenitor cells (CVPCs) was reported in severe ischemic heart failure patients; however, the safety and efficacy of these novel treatments must be thoroughly investigated in large animal models, especially in nonhuman Primate (NHP) models, before larger clinical trials can be initiated. Because these studies necessarily require the transplantation of human cells into nonhuman species, many of the transplanted cells may be rejected by the immune system of animals, especially for studies performed in nonhuman pri-mates. Thus, concomitant immune suppression therapy must be maintained throughout the study period, but the relative effectiveness and safety of many immunosuppressive agents, or combinations of agents, have rarely been the subject of formal investigation. Furthermore, the mechanism whereby hPSC-CVPCs improve heart function (direct remuscularization or paracrine effects) after MI needs to be clarified, especially in clinical relevant NHP models.
Recently, Chinese researchers compared cyclosporine alone with a combination of cyclosporine, methylprednisolone, and the CD25 antibody Simulect (multiple-drug regimen [MDR]) in cynomolgous monkeys that were transplanted with hPSC-CVPCs into myocardium with experimentally induced acute MI.
Compared to cyclosporine alone, MDR attenuates immune rejection and improves survival of hPSC-CVPCs in primates; this is associated with less apoptosis of native cardiac cells and better recovery of left ventricular function at 28 days. However, even with MDR, transplanted hPSC-CVPCs do not engraft and do not survive at 140 days after transplantation, indicating paracrine effects contribute to the benefits of hPSC-CVPCs but not remuscularization in infarct repair. This is the largest study of hPSCs in non-human primates in cardiovascular field so far (n=32). The findings provide important knowledge for the translational research in cardiac repair of infarcted hearts by cell therapy.
This work was completed by research teams led by Dr. WANG Jian’an and Dr. HU Xinyang from the Second Affiliated Hospital of Zhejiang University collaborated with Dr. YANG Huangtian’s team from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The results entitled “Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates” was published online in Circ Res on Jan 17, 2018.
The study was funded by Ministry of Science and Technology of China, National Natural Science Foundation of China, and Chinese Academy of Sciences, etc.
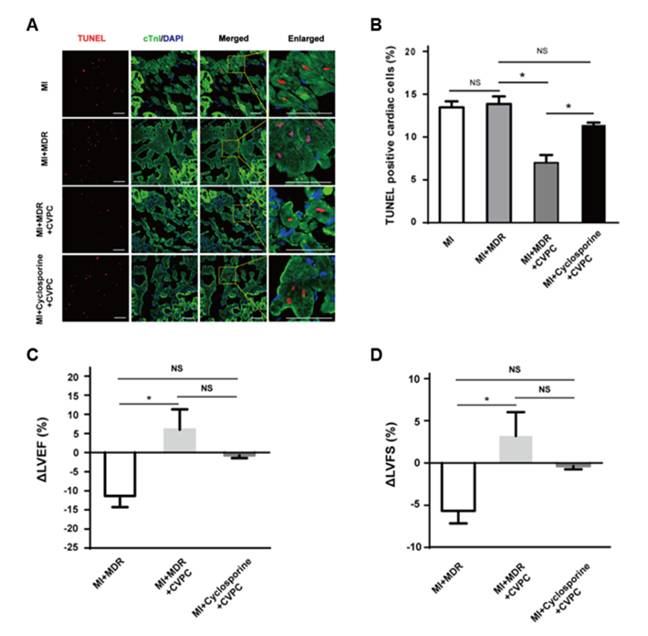
Figure: Functional improvement following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates.
(Image by WANG Jian’an, HU Xinyang and YANG Huangtian)
Dr. HU Xinyang
Department of Cardiology
Provincial Key Lab of Cardiovascular Research
Second Affiliated Hospital
Zhejiang University School of Medicine
Hangzhou 310009, China
E-mail: hxy0507@126.com
Dr. WANG Jian'an
Department of Cardiology
Provincial Key Lab of Cardiovascular Research
Second Affiliated Hospital
Zhejiang University School of Medicine
Hangzhou 310009, China
E-mail: Jian_an_wang@yahoo.com
Prof. YANG Huang-Tian
Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
320 Yueyang Road, Shanghai 200031, China.
E-mail: htyang@sibs.ac.cn
