- Home >> ALL News >> Highlights
SETD2-mediated EZH2 Methylation Restricts Prostate Cancer Metastasis
Prostate cancer (PCa) remains a leading cause of cancer death in men worldwide. Although patients with localized tumors have favorable outcomes, the 5-year survival for metastatic patients is extremely poor. Therefore, it is of great interest to identify the mechanism driving PCa metastasis.
The histone H3K36 methyltransferase SETD2 is frequently mutated or deleted in a variety of human tumors, such as clear cell renal cell carcinoma (ccRCC), glioma, colorectal cancer, and PCa. Nevertheless, the role of SETD2 loss in oncogenesis remains largely undefined. It has long been observed that the level of SETD2-mediated H3K36me3 showed a significant inverse correlation with that of EZH2-catalyzed H3K27me3. However, it remains unclear how the interplay between SETD2 and EZH2 is achieved and what is the pathological implications.
On July 2, 2020, the international academic journal Cancer Cell published the research paper "SETD2 Restricts Prostate Cancer Metastasis by Integrating EZH2 and AMPK Signaling Pathways" by Dr. QIN Jun's group from Shanghai Institute of Nutrition and Health (SINH) of Chinese Academy of Sciences (CAS) and collaborators. This study reveals that H3K36 methyltransferase SETD2 curtails the level of EZH2 to inhibit prostate cancer metastasis by sensing extracellular energy states.
This study established that SETD2 loss potentiated PCa metastasis. Mechanistic studies showed that SETD2-mediated EZH2 methylation at K735 residue facilitates EZH2 destruction, and prevents the cells transforming into a high H3K27me3 chromatin state to develop full-blown metastatic PCa.
Furthermore, the researchers demonstrated that the function of SETD2 in PCa is largely dependent on the methylation of EZH2 at K735. Besides, they found a patient-derived hotspot mutation SETD2-R1523 in human cancers database. Cancer associated SETD2 hotspot mutation at R1523 residue disrupt the interaction and methylation of EZH2 to drive tumor progression in H3K36 methyltransferase independent manner. They further indicated that AMPK signaling regulates SETD2 expression, suggesting that the SETD2-EZH2 axis translates metabolic cues to alter cellular chromatin state. This finding is of translational interest as metformin may inhibit metastasis caused by SETD2 downregulation.
Together, these results demonstrate that the SETD2-EZH2 axis integrates metabolic and epigenetic signaling to restrict PCa metastasis.
The graduate students YUAN Huairui, HAN Ying and WANG Xuege from SINH are the co-first authors of this study. Prof. QIN Jun is the corresponding author of this article. JIANG Jun, a chief physician from Daping Hospital of Army Medical University, and Prof WANG Xiaoming from Nanjing Medical University are the co-corresponding authors. This work was greatly assisted by Prof. TAN Minjia from Shanghai Institute of Materia Medica, CAS, Prof. ZHANG Jian, LI Li from Shanghai Jiao Tong University School and Prof. LI Qintong from Sichuan University. This study was supported by grants from the National Key Research and Development Program of China, the National Natural Science Foundation, and CAS.
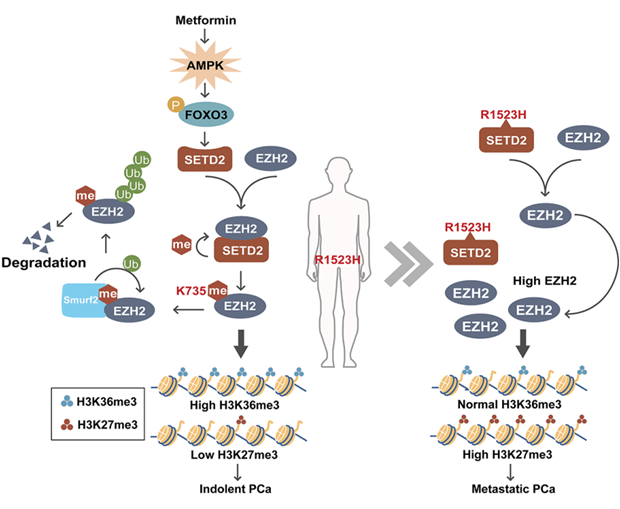
Schematic illustration of the proposed model for SETD2 in PCa metastasis. (Image provided by Dr. QIN Jun's group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn