- Home >> ALL News >> Highlights
Scientists Jointly Reveal Novel Oncogenic Driver Gene in Human Gastrointestinal Stromal Tumors
A research article describing a novel druggable driver gene in one of the human deadly cancers, entitled “Mutational Inactivation of mTORC1 Repressor Gene DEPDC5 in Human Gastrointestinal Stromal Tumors” was published online in Proc Natl Acad Sci USA on Oct 21, 2019.
Sarcomas, cancers that arise from transformed cells of mesenchymal (connective tissue) original, are quite deadly. Gastrointestinal stromal tumors (GISTs) are the most common human sarcoma and are initiated by activating mutations in the KIT receptor tyrosine kinase. Although sharing the same KIT mutations, micro-GISTs have a limited growth potential and hence are restrained at subcentimetre level. The fact that micro-GISTs are common in general individuals, found in one third of the general population, without clinical symptoms indicates additional genetic alterations contribute to the progression of clinical GISTs. Chromosome 22q deletions are frequent chromosomal abnormalities in human GISTs, occurring in ~50% GISTs, and are thought to contribute to the pathogenesis of this disease. However, the crucial gene in 22q has been unknown for decades.
To identify the causative gene in chromosome 22q, a translational research team led by Prof. WANG Yuexiang at the Shanghai Institute of Nutrition and Health (SINH) of Chinese Academy of Sciences performed whole exome sequencing and reported recurrent genomic inactivated DEPDC5 gene mutations in GISTs. They showed that DEPDC5 is a chromosome 22q-targeting tumor suppressor, silenced by mutations in GIST specifically.
The team further provided evidence that DEPDC5 inactivation promotes GIST cell proliferation through activating mTORC1 signaling pathway and subsequently inhibits cell cycle arrest. Finally, the team demonstrated that DEPDC5 modulates the sensitivity of GIST to KIT inhibitors, and the combination therapy with mTOR inhibitor and KIT inhibitor may work better in GIST patients with DEPDC5 inactivation.
These findings of recurrent genomic alterations, together with functional data, validate the DEPDC5 as a bona fide tumor suppressor contributing to GIST progression and a biologically relevant target of the frequent chromosome 22q deletions. The DEPDC5 inactivated mutations are prognostic in that they are associated with aggressive GISTs where they promote GIST progression and reduce sensitivity to KIT inhibitors. The intriguing discovery of DEPDC5 also shows that biologic mechanisms of focal epilepsy (genetic DEPDC5 inactivation is responsible for focal epilepsy) are relevant in tumorigenesis, and thereby highlight that recent therapeutic developments for focal epilepsy (eg DEPDC5 agonists) could also serve as anti-cancer drugs.
The research was led by Prof. WANG Yuexiang from SINH, together with Prof. Jonathan Fletcher from Brigham and Women’s Hospital and Harvard Medical School, and supported by Prof. CAO Hui and Dr. SHEN Yanying from Renji Hospital, Prof. WANG Chunmeng from Fudan University Cancer Center, Dr. HUANG Tao from SINH, Prof. WANG Linhui from Changzheng Hospital, and Prof. HE Yi from No. 1 Hospital of Jiaxing. This project was financially supported by National Natural Science Foundation of China, Ministry of Science and Technology of China, Science and Technology Commission of Shanghai Municipality, and Chinese Academy of Sciences.
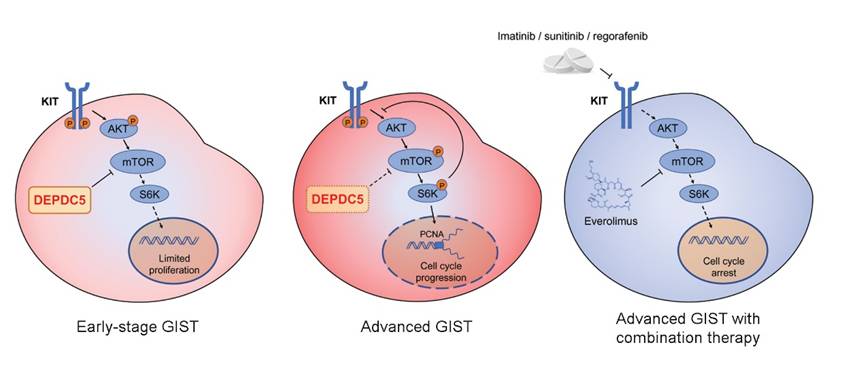
DEPDC5 inactivated mutations promote gastrointestinal stromal tumor (GIST) progression. (Image provided by Prof. Wang's team)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health
Chinese Academy of Sciences
Email: sibssc@sibs.ac.cn